Multispecies characterization of immature neurons in the mammalian amygdala reveals their expansion in primates
- PMID: 40811712
- PMCID: PMC12370197
- DOI: 10.1371/journal.pbio.3003322
Multispecies characterization of immature neurons in the mammalian amygdala reveals their expansion in primates
Abstract
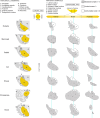
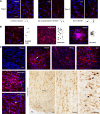
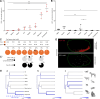
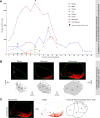
Structural changes involving new neurons can occur through stem cell-driven neurogenesis, and through incorporation of late-maturing "immature" neurons into networks, namely undifferentiated neuronal precursors frozen in a state of arrested maturation. The latter have been found in the cerebral cortex and are particularly abundant in large-brained mammals, covarying with the size of the brain and cortex. Similar cells have been described in the amygdala of some species, although their features and interspecies variation remain poorly understood. Here, their occurrence, number, morphology, molecular expression, age-related changes, and anatomical distribution in amygdala subdivisions were systematically analyzed in eight diverse mammalian species (including mouse, naked mole rat, rabbit, marmoset, cat, sheep, horse, and chimpanzee) widely differing in neuroanatomy, brain size, life span, and socioecology. We identify converging evidence that these amygdala cells are immature neurons and show marked phylogenetic variation, with a significantly greater prevalence in primates. The immature cells are largely located within the amygdala's basolateral complex, a region that has expanded in primate brain evolution in conjunction with cortical projections. In addition, amygdala immature neurons also appear to stabilize in number through adulthood and old age, unlike other forms of plasticity that undergo marked age-related reduction. These results support the emerging view that large brains performing complex socio-cognitive functions rely on wide reservoirs of immature neurons.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Phylogenetic variation of immature neurons in mammalian amygdala: high prevalence in primate expanded nuclei projecting to neocortex.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.630481. doi: 10.1101/2024.12.27.630481. bioRxiv. 2024. PMID: 39764019 Free PMC article. Preprint.
-
Phylogenetic variation of layer II cortical immature neurons in dog and horse confirms covariance with brain size and neocortical surface.Brain Struct Funct. 2025 Jul 7;230(6):115. doi: 10.1007/s00429-025-02981-x. Brain Struct Funct. 2025. PMID: 40622463 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Gómez-Climent MA, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, et al. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex. 2008;18:2229–40. - PubMed
-
- König R, Benedetti B, Rotheneichner P, O′Sullivan A, Kreutzer C, Belles M, et al. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: a local reservoir for adult cortical neuroplasticity? Front Biol. 2016;11:193–213.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

