The transmission blocking activity of artemisinin-combination, non-artemisinin, and 8-aminoquinoline antimalarial therapies: A pooled analysis of individual participant data
- PMID: 40811722
- PMCID: PMC12352847
- DOI: 10.1371/journal.pmed.1004683
The transmission blocking activity of artemisinin-combination, non-artemisinin, and 8-aminoquinoline antimalarial therapies: A pooled analysis of individual participant data
Abstract
Background: Interrupting human-to-mosquito transmission is important for malaria elimination strategies as it can reduce infection burden in communities and slow the spread of drug resistance. Antimalarial medications differ in their efficacy in clearing the transmission stages of Plasmodium falciparum (gametocytes) and in preventing mosquito infection. Here, we present a retrospective combined analysis of six trials conducted at the same study site with highly consistent methodologies that allows for a direct comparison of the gametocytocidal and transmission-blocking activities of 15 different antimalarial regimens or dosing schedules.
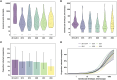
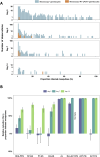
Methods and findings: Between January 2013 and January 2023, we conducted six clinical trials evaluating antimalarial treatments with transmission endpoints at the Clinical Research Centre of the Malaria Research and Training Centre of the University of Bamako in Mali. These trials tested Artemisinin-Combination Therapies (ACTs), non-ACT regimens and combinations with 8-aminoquinolines. Participants were males and non-pregnant females, between 5 and 50 years of age, who presented with P. falciparum mono-infection and gametocyte carriage by microscopy. We collected blood samples before and after treatment for thick film microscopy, infectivity assessments by mosquito feeding assays and molecular quantification of gametocytes. To combine direct and indirect effects of treatment groups across studies, we performed a network meta-analysis. This analysis quantified changes in mosquito infection rates and gametocyte densities within treatment groups over time and between treatments. In a pooled analysis of 422 participants, we observed substantial differences between antimalarials in gametocytocidal and transmission-blocking activities. Artemether-lumefantrine (AL) was significantly more potent at reducing mosquito infection rates within 48 h than dihydroartemisinin-piperaquine (p = 0.0164) and sulfadoxine-pyrimethamine plus amodiaquine (p = 0.0451), while this difference was near-significant for artesunate-amodiaquine (p = 0.0789) and pyronaridine-artesunate (p = 0.0519). The addition of single low-dose primaquine (SLD PQ) accelerated gametocyte clearance for any ACT and led to a substantially greater reduction in mosquito infection rate within 48 h of treatment for all ACTs except AL, while an SLD of the 8-aminoaquinoline tafenoquine showed a delayed activity, compared to SLD PQ, but was similarly effective. The main limitations of the study include the inclusion of highly infectious individuals, which may not reflect the broader malaria patient population with lower or undetectable gametocyte densities and the small sample sizes in some treatment groups, which resulted in wide confidence intervals and reduced the certainty of effect estimates.
Conclusions: We found marked differences among ACTs and single low-dose 8-aminoquinoline drugs in their ability and speed to block transmission. The findings from this analysis can support treatment policy decisions for malaria elimination and be integrated into mathematical models to improve the accuracy of predictions regarding community transmission and the spread of drug resistance under varying treatment guidelines.
Copyright: © 2025 Vanheer et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: CD is a member of the Malaria Policy Advisory Group (MPAG). TB contributed to a Guideline Development Group of the World Health Organization for recommendations for the final phase of elimination and prevention of re-establishment of malaria.
Figures




References
-
- Stepniewska K, Humphreys GS, Gonçalves BP, Craig E, Gosling R, Guerin PJ, et al. Efficacy of single-dose primaquine with artemisinin combination therapy on Plasmodium falciparum gametocytes and transmission: an individual patient meta-analysis. J Infect Dis. 2022;225(7):1215–26. doi: 10.1093/infdis/jiaa498 - DOI - PMC - PubMed
-
- Bolscher JM, Koolen KMJ, van Gemert GJ, van de Vegte-Bolmer MG, Bousema T, Leroy D, et al. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J Antimicrob Chemother. 2015;70(5):1357–66. doi: 10.1093/jac/dkv003 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

