Elicitation of neutralizing antibodies and IgG4 subclass switching following booster vaccination with ancestral COVID-19 mRNA vaccines does not reduce breakthrough infections
- PMID: 40812315
- PMCID: PMC12355716
- DOI: 10.1080/21645515.2025.2547517
Elicitation of neutralizing antibodies and IgG4 subclass switching following booster vaccination with ancestral COVID-19 mRNA vaccines does not reduce breakthrough infections
Abstract
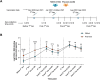
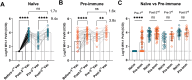
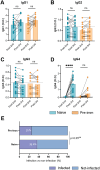
Vaccination with COVID-19 mRNA vaccines generates robust antibody responses, but the impact of prior infection on the quality of these responses, particularly immunoglobulin G (IgG) subclass profiles remain unclear. A longitudinal study was conducted to compare humoral immune responses in SARS-CoV-2 infection-naïve and pre-immune (previously infected) individuals following a two-dose mRNA vaccine primary series and a booster dose. Anti-spike receptor-binding domain (RBD) IgG levels, neutralizing antibody titers against the vaccine-matched (Wuhan-Hu-1) virus and the Omicron BA.1 variant, and IgG1-IgG4 subclass distributions over time were measured. After two doses, pre-immune participants had higher anti-RBD IgG (2-fold, p < .001) and neutralization titers than naïve participants (GMT±SD; 6407 ± 4 vs 5706 ± 3.5). Notably, 89.7% of pre-immune versus 28.1% of naïve sera neutralized Omicron BA.1 (p < .0001). However, a third (booster) raised antibody levels in naïve participants to a statistically similar titer to pre-immune participants (p > .05). The booster vaccination also markedly enhanced the titers of cross-neutralizing antibodies against Omicron BA.1 in both vaccine groups. This increased the proportion of responders from 31.8% to 95.5% in naïve participants. Booster vaccinations induced significant IgG4 titers in naïve (p < .0001), but not in pre-immune participants (p > .05) compared to primary series of vaccination levels. Both vaccinated naïve participants and pre-immune vaccinated participants had a breakthrough infection within one year following the booster vaccination (36.4% vs 25%, p > .05 respectively). We report that the presence of IgG4 antibodies, specifically in naïve individuals, did not alter in vitro virus neutralizing response against ancestral WH-1 and Omicron BA.1 variant, with comparable breakthrough infection rates.
Keywords: COVID-19; IgG subclass switching; SARS-CoV-2; mRNA vaccine; naïve; pre-immune; virus neutralization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against,COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. - DOI - PMC - PubMed
-
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP.. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
-
- Lapadula G, Mezzadri L, Lo Cascio G, Antolini L, Malandrin S, Ranzani A, Limonta S, Cavallero A, Bonfanti P. Anti-spike antibody level is associated with the risk of clinical progression among subjects hospitalized with COVID-19 pneumonia: results from a retrospective cohort study. Infection. 2024;52(4):1499–1509. doi: 10.1007/s15010-024-02250-9. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
