ReSTech project on Xiaomi wearable devices for monitoring and detecting obstructive sleep apnoea: observational study protocol
- PMID: 40812810
- PMCID: PMC12352195
- DOI: 10.1136/bmjopen-2025-101824
ReSTech project on Xiaomi wearable devices for monitoring and detecting obstructive sleep apnoea: observational study protocol
Abstract
Introduction: Sleep-related breathing disorders have become a significant public health concern due to their negative impact on the population's quality of life and overall health. Despite being underdiagnosed, their prevalence has increased in recent years, particularly in cases of obstructive sleep apnoea (OSA). Early diagnosis and detection of OSA are essential for timely treatment to mitigate the physical and health consequences. While polysomnography remains the gold standard for diagnosis, its limitations have led to the adoption of nocturnal polygraphy as an alternative for diagnosis. The scientific community is seeking devices that enable continuous monitoring of sleep status and other relevant parameters in this population. This study aims to analyse a wearable device as a complementary tool for monitoring health status and daily activity in people with potential OSA.
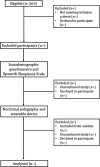
Methods and analysis: This observational and cross-sectional study will be conducted at the Sleep Respiratory Disorders and Home Ventilation Unit of a Hospital Álvaro Cunqueiro in Vigo. The aim is to recruit 246 participants who meet the inclusion criteria. Specific statistical methods will be employed to evaluate the accuracy and quality of the data collected by the Xiaomi Mi Smart Band 9.
Ethics and dissemination: This protocol study has been approved by the Pontevedra-Vigo Ourense Research Ethics Committee (process number 2024/260). All participants will sign a statement of informed consent. Study results will be disseminated in peer-reviewed journal articles.
Trial registration number: NCT06606691.
Keywords: Health; Health informatics; Quality of Life; eHealth.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Drug therapy for obstructive sleep apnoea in adults.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003002. doi: 10.1002/14651858.CD003002.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2013 May 31;(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. PMID: 16625567 Updated.
-
Multilevel-Single Stage-Functional Rhinoplasty & BRP (Barb Reposition Palatoplasty) in Surgical Management of Primary Snorers, UARS and Mild OSA.Indian J Otolaryngol Head Neck Surg. 2024 Dec;76(6):5672-5681. doi: 10.1007/s12070-024-05059-y. Epub 2024 Oct 8. Indian J Otolaryngol Head Neck Surg. 2024. PMID: 39559159
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Drug therapy for obstructive sleep apnoea in adults.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. Cochrane Database Syst Rev. 2013. PMID: 23728641 Free PMC article.
References
-
- L Mandel H, Colleen G, Abedian S, et al. Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: an electronic health record-based analysis from the RECOVER initiative. Sleep. 2023;46:zsad126. doi: 10.1093/sleep/zsad126. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical