Single low-dose primaquine for malaria control in Africa: a systematic review of safety, efficacy and implementation barriers
- PMID: 40813102
- PMCID: PMC12352169
- DOI: 10.1136/bmjgh-2025-020264
Single low-dose primaquine for malaria control in Africa: a systematic review of safety, efficacy and implementation barriers
Abstract
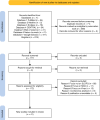
Since 2012, the WHO has recommended a single low dose of primaquine (SLDPQ, 0.25 mg/kg) alongside artemisinin-based combination therapies (ACTs) to block Plasmodium falciparum transmission and combat artemisinin resistance. Despite its proven benefits, SLDPQ adoption in African malaria policies remains limited. We conducted a systematic review of studies published between 2012 and 2023 on the safety, efficacy and implementation of SLDPQ in Africa. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we searched 7 databases and screened 819 records. Eligible studies focused on SLDPQ co-administered with ACTs for treating uncomplicated P. falciparum malaria in African contexts. Data were extracted and analysed from 41 studies, including 15 randomised controlled trials (RCTs) and 26 non-trial studies. SLDPQ was found to be safe and well-tolerated, including in glucose-6-phosphate dehydrogenase deficiency individuals and children under 5. Eight RCTs confirmed significant reductions in gametocyte carriage, validating SLDPQ's individual-level efficacy. However, evidence on community-level impact remains limited. Key implementation barriers include persistent misconceptions about primaquine toxicity, absence of paediatric formulations and operational challenges in health systems. Most studies used the WHO-recommended dose (0.25 mg/kg), but higher doses and age-based regimens were also investigated. This review supports SLDPQ as a safe and effective tool for malaria transmission reduction in Africa. Addressing barriers to implementation, through health worker training, community sensitisation and operational research, is essential to accelerate its adoption. The ongoing Implementing Primaquine Single Low Dose in Africa project aims to generate real-world evidence across three countries, with a focus on paediatric use and health system integration. SLDPQ scale-up should be prioritised within malaria elimination strategies across sub-Saharan Africa.
Keywords: Decision Making; Epidemiology; Health policies and all other topics; Malaria.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- World Health Organization World malaria report. 2024
-
- Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
