Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: a randomised, double-blind, placebo-controlled, phase II study
- PMID: 40813108
- PMCID: PMC12352177
- DOI: 10.1136/rmdopen-2025-005557
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: a randomised, double-blind, placebo-controlled, phase II study
Abstract
Background: Iscalimab (CFZ533) is a novel, anti-CD40 monoclonal antibody. This study evaluated the efficacy, pharmacokinetics and safety of iscalimab versus placebo as add-on to standard-of-care (SoC) therapy in patients with biopsy-proven active proliferative lupus nephritis (LN).
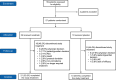
Methods: This was a phase II, randomised, double-blind, placebo-controlled, multicentre study including patients with a diagnosis of systemic lupus erythematosus with active LN. Patients were randomly assigned (2:1) to receive either intravenous iscalimab (10 mg/kg) or placebo for 24 weeks on top of SoC for LN. The primary efficacy endpoint was the ratio from baseline in urinary protein-to-creatinine ratio (UPCR) at week 24. Safety assessments included adverse events (AEs) and serious AEs (SAEs) during treatment and follow-up up to 49 weeks.
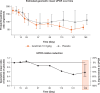
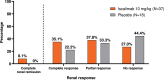
Findings: Of the 57 patients (iscalimab, n=39; placebo, n=18) randomised, 31 (54.4%) completed the study. The primary efficacy endpoint was met: at week 24, the relative improvement from baseline in proteinuria (UPCR) was 63.1% and 36.3% in the iscalimab and placebo arms, respectively. UPCR to baseline at week 24 showed a statistically significant reduction of 42.1% in the iscalimab versus placebo arm. Most AEs were of mild to moderate severity in both treatment arms. Overall, seven SAEs were reported in six patients (15.4%) in the iscalimab arm versus four in three patients (16.7%) in the placebo arm.
Interpretation: Iscalimab showed a significant improvement in proteinuria (UPCR) in patients with active LN. Iscalimab was generally well tolerated with the exception of a few severe infections and one case of macrophage-activation syndrome in immunosuppressed and comorbid patients.
Trial registration number: NCT03610516.
Keywords: Autoimmune Diseases; Biological Therapy; Glucocorticoids; Lupus Erythematosus, Systemic; Lupus Nephritis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: NS has no potential competing interests. JW-M received consulting fees from GSK and AstraZeneca; payment or honoraria from GSK, AstraZeneca, Otsuka and Alexion; travel assistance or support for attending meetings from Novartis, AstraZeneca and Otsuka; participated on the data safety monitoring or advisory board of GSK, AstraZeneca, Otsuka, Alexion and Novartis. JW-M’s institution received grants or contracts from Novartis towards study site payment. JW-M is a Spokesperson of the German association of nephrology and member of the AWMF. AM received consulting fees from GSK, Roche, Kezar, Biogen, Bristol Myers Squibb and Pfizer; payment or honoraria from GSK, Roche, Biogen, Bristol Myers Squibb and Pfizer; participated on the data safety monitoring or advisory board of GSK, Roche, Kezar, Biogen, Bristol Myers Squibb and Pfizer. MW, RD, CS, JR, RF, PG and TS are employees of Novartis. AS-R was an employee of Novartis at the time of conduct of this study.
Figures

Similar articles
-
Efficacy and safety of guselkumab in patients with active lupus nephritis: results from a phase 2, randomized, placebo-controlled study.Rheumatology (Oxford). 2025 May 1;64(5):2731-2740. doi: 10.1093/rheumatology/keae647. Rheumatology (Oxford). 2025. PMID: 39673415 Free PMC article. Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Efficacy and Safety of Obinutuzumab in Active Lupus Nephritis.N Engl J Med. 2025 Apr 17;392(15):1471-1483. doi: 10.1056/NEJMoa2410965. Epub 2025 Feb 7. N Engl J Med. 2025. PMID: 39927615 Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
