Dysregulation of GTPase-activating protein-binding protein1 in the pathogenesis of metabolic dysfunction-associated steatotic liver disease
- PMID: 40813380
- PMCID: PMC12354899
- DOI: 10.1038/s41467-025-63022-z
Dysregulation of GTPase-activating protein-binding protein1 in the pathogenesis of metabolic dysfunction-associated steatotic liver disease
Abstract
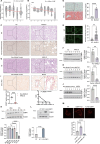
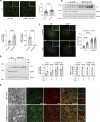
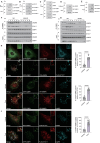
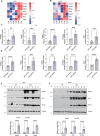
Metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) are two common liver disorders characterized by abnormal lipid accumulation. Our study found reduced levels of GTPase-activating protein-binding protein1 (G3BP1) in patients with MASLD and MASH, suggesting its involvement in these liver disorders. Hepatocyte-specific G3BP1 knockout (G3BP1 HKO) male mice had more severe MASLD and MASH than their corresponding controls. Intriguingly, the G3BP1 HKO MASLD model male mice exhibit dysregulated autophagy, and biochemical analyses demonstrated that G3BP1 promotes autophagosome-lysosome fusion through direct interactions with the SNARE proteins STX17 and VAMP8. We also show that hepatic knockout of G3BP1 promotes de novo lipogenesis, and ultimately found that G3BP1 is required for the nuclear translocation of the well-known liver-lipid-regulating transcription factor TFE3. Taken together, our results suggest that G3BP1 should be investigated as a potential target for developing medical interventions to treat MASLD and MASH.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol.29, 101133 (2024). - PubMed
-
- Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol.10, 686–690 (2013). - PubMed
-
- Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol.10, 330–344 (2013). - PubMed
-
- Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol.62, S47–S64 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

