IPaulHet - spatially-defined, wingtip-flexible, N,C-chelating oxazole and thiazole donor N-heterocyclic carbene ligands
- PMID: 40813567
- PMCID: PMC12360485
- DOI: 10.1039/d5dt01576f
IPaulHet - spatially-defined, wingtip-flexible, N,C-chelating oxazole and thiazole donor N-heterocyclic carbene ligands
Abstract
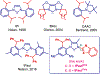
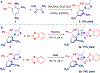
N-Heterocyclic carbenes (NHCs) are among the most versatile ligands in transition metal catalysis with their steric and electronic properties playing a critical role in governing reactivity and selectivity. In this area, the wingtip unsymmetrical IPaul ligand introduced by Nelson and co-workers offers a unique balance of steric bulk and flexibility characterized by spatially-defined steric features. Herein, we report a new class of IPaul-based ligands bearing benzoxazole and benzothiazole donor wingtips. The synthesis, application, and structural and electronic characterization are described. These ligands retain the defining 'bulky yet flexible' profile of IPaul, while enabling precise control over the catalytic pocket geometry through N-heteroaryl wingtip substitution. We present their coordination chemistry with Ag(I), Pd(II), Rh(I), and Se as well as catalytic studies in cross-coupling and hydrosilylation catalyzed by Ag, Pd, and Rh complexes. By combining the steric asymmetry of IPaul with the chelating flexibility of N-azole donors, this ligand class provides stabilization of reactive metal centers and is well-suited for diverse catalytic applications. We anticipate that the combination of steric flexibility with N,C-chelation in versatile N-heterocyclic carbenes will be of broad interest across organometallic, inorganic and catalytic chemistry.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures



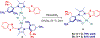
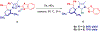
Similar articles
-
Induced-Fit Chiral N-Heterocyclic Carbene Ligands for Asymmetric Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2157-2177. doi: 10.1021/acs.accounts.5c00304. Epub 2025 Jun 19. Acc Chem Res. 2025. PMID: 40536020
-
IPr*Oxa - a new class of sterically-hindered, wingtip-flexible N,C-chelating oxazole-donor N-heterocyclic carbene ligands.Dalton Trans. 2023 Oct 3;52(38):13608-13617. doi: 10.1039/d3dt02255b. Dalton Trans. 2023. PMID: 37698540
-
IPr**(4-Bp)-Highly Hindered, Ring Extended N-Heterocyclic Carbenes.Organometallics. 2025 Aug 7;44(16):1848-53. doi: 10.1021/acs.organomet.5c00232. Online ahead of print. Organometallics. 2025. PMID: 40837034 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Bourissou D, Guerret O, Gabbaï FP and Bertrand G, Chem. Rev, 2000, 100, 39–92; - PubMed
- (b) For the original study by Arduengo, see: Arduengo AJ III, Harlow RL and Kline M, J. Am. Chem. Soc, 1991, 113, 361–363;
- (c) Arduengo AJ III, Acc. Chem. Res, 1999, 32, 913–921.
-
- Hopkinson MN, Richter C, Schedler M and Glorius F, Nature, 2014, 510, 485–496; - PubMed
- (b) Nolan SP, N-Heterocyclic Carbenes, Wiley, 2014;
- (c) Diez-Gonzalez S, N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, RSC, 2016;
- (d) Huynh HV, The Organometallic Chemistry of N-Heterocyclic Carbenes, Wiley, 2017;
- (e) Cazin CSJ, N-Heterocyclic Carbenes in Transition Metal Catalysis, Springer, 2011;
- (f) Kantchev EAB, O’Brien CJO and Organ MG, Angew. Chem., Int. Ed, 2007, 46, 2768–2813; - PubMed
- (g) Hermann WA, Angew. Chem., Int. Ed, 2002, 41, 1290–1309; - PubMed
- (h) Peris E, Chem. Rev, 2018, 118, 9988–10031; - PubMed
- (i) Sipos G and Dorta R, Coord. Chem. Rev, 2018, 375, 13–68;
- (j) Iglesias M and Oro LA, Chem. Soc. Rev, 2018, 47, 2772–2808; - PubMed
- (k) Danopoulos AA, Simler T and Braunstein P, Chem. Rev, 2019, 119, 3730–3961; - PubMed
- (l) Zhao Q, Meng G, Nolan SP and Szostak M, Chem. Rev, 2020, 120, 1981–2048; - PMC - PubMed
- (m) Chen C, Liu FS and Szostak M, Chem. – Eur. J, 2021, 27, 4478–4499; - PMC - PubMed
- (n) Jazzar R, Soleilhavoup M and Bertrand G, Chem. Rev, 2020, 120, 4141–4168; - PubMed
- (o) Morvan J, Mauduit M, Bertrand G and Jazzar R, ACS Catal, 2021, 11, 1714–1748;
- (p) Gao P and Szostak M, Coord. Chem. Rev, 2023, 485, 215110. - PMC - PubMed
-
- Yus M, Najcra C, Foubelo F and Sansano JM, Chem. Rev, 2023, 123, 11817–11893; - PMC - PubMed
- (b) John A and Ghosh P, Dalton Trans, 2010, 39, 7183–7206; - PubMed
- (c) Diez-Gonzalez S and Nolan SP, Coord. Chem. Rev, 2007, 251, 874–883;
- (d) Dröge T and Glorius F, Angew. Chem., Int. Ed, 2010, 49, 6940–6952. - PubMed
-
- Koy M, Bellotti P, Das M and Glorius F, Nat. Catal, 2021, 4, 352–363.
Grants and funding
LinkOut - more resources
Full Text Sources

