Unraveling the impact of crizotinib to promote megakaryopoiesis for alleviating thrombocytopenia in myelodysplastic neoplasms
- PMID: 40813622
- PMCID: PMC12589124
- DOI: 10.1038/s41375-025-02729-w
Unraveling the impact of crizotinib to promote megakaryopoiesis for alleviating thrombocytopenia in myelodysplastic neoplasms
Abstract
Current therapeutic options for myelodysplastic neoplasms (MDS)-associated thrombocytopenia are limited. Megakaryocyte maturation might be an innovative therapeutic strategy because its dysregulation profoundly contributes to MDS pathogenesis. Here, we identified crizotinib, a clinically approved anti-cancer drug for anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer, as a potent inducer of megakaryocyte maturation. We demonstrated that crizotinib effectively induced polyploidization to increase the platelet-producing capacity of megakaryocytes derived from an MDS murine model and MDS patients by targeting Aurora kinases rather than its canonical targets, ALK/ROS1/c-MET. Importantly, crizotinib administration substantially ameliorated thrombocytopenia in our preclinical model. Our findings underscore the remarkable potential of crizotinib for drug repurposing and offer a novel therapeutic strategy for MDS patients with thrombocytopenia facing health-related quality of life concerns.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: HK and HH filed a patent for applications of therapeutic compounds for hematological disorders, including those described in this study. Patent names: “Therapeutic compounds for hematological disorders.” Patent applicant: Tokyo University of Pharmacy and Life Sciences. Name of inventors: HK and HH. Patent number: Japanese Patent Application No. 2024-228529. Patent status: pending. Specific aspect of the manuscript covered in the patent application: The patent application covers the application of crizotinib to increase platelet counts in MDS patients. HK received a research grant from Nippon Shinyaku Co., Ltd. HH has received advisory board fees from Novartis. The other authors have no conflicts of interest to declare.
Figures
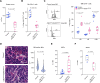

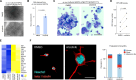
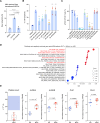

References
-
- Platzbecker U, Wong RS, Verma A, Abboud C, Araujo S, Chiou TJ, et al. Safety and tolerability of eltrombopag versus placebo for treatment of thrombocytopenia in patients with advanced myelodysplastic syndromes or acute myeloid leukaemia: a multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial. Lancet Haematol. 2015;2:e417–26. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- JP23K06899/MEXT | Japan Society for the Promotion of Science (JSPS)
- U54 HG006097/HG/NHGRI NIH HHS/United States
- JP20K07840/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K10365/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23H05473/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

