Anti-PD-1 therapy in unresectable desmoplastic melanoma: the phase 2 SWOG S1512 trial
- PMID: 40813711
- PMCID: PMC12419231
- DOI: 10.1038/s41591-025-03875-5
Anti-PD-1 therapy in unresectable desmoplastic melanoma: the phase 2 SWOG S1512 trial
Erratum in
-
Publisher Correction: Anti-PD-1 therapy in unresectable desmoplastic melanoma: the phase 2 SWOG S1512 trial.Nat Med. 2025 Nov;31(11):3933. doi: 10.1038/s41591-025-03975-2. Nat Med. 2025. PMID: 40858972 No abstract available.
Abstract
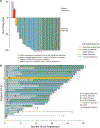
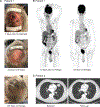
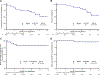
Desmoplastic melanoma is a distinct subtype of melanoma known to have preexisting immune infiltrates and high ultraviolet light damage, resulting in a high tumor mutational burden. We hypothesized that this may result in high response rates with single-agent anti-programmed death protein 1 (PD-1) therapy. SWOG S1512 was a two-cohort clinical trial testing the activity of pembrolizumab in patients with surgically resectable (cohort A) and unresectable (cohort B) desmoplastic melanoma. Here we report on the cohort B single-arm clinical trial, which enrolled 27 patients with unresectable desmoplastic melanoma receiving pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years, with the primary endpoint of complete response rate. The complete response rate was 37% (95% confidence interval: 19-58%), and the post hoc endpoint of objective response rate was 89% (95% confidence interval: 71-98%). The estimated secondary endpoints of 3-year melanoma-specific progression-free survival and overall survival were 84% and 96%, respectively, with only one patient having died from melanoma progression. Ten patients (37%) experienced grade 3 or 4 adverse events, and nine patients (33%) discontinued treatment because of adverse events. Patients with advanced desmoplastic melanoma have a high response rate to single-agent PD-1 blockade therapy, supporting single-agent anti-PD-1 as the treatment of choice, but are limited by a frequency of toxicities that is numerically higher than in other patient populations. ClinicalTrials.gov identifier: NCT02775851.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: K.L.K.: institutional research support from Bristol Myers Squibb and trial support from GlaxoSmithKline, Immunocore, Varian Medical Systems and Merck. S.L.B. reports no competing interests. Z.E.: advisory board for Regeneron, Pfizer, Replimune, Incyte, Natera and SunPharma and research funding from Pfizer and Boehringer Ingelheim. S.H.-L.: scientific advisor/consultant for Amgen, Ascendis, Astellas, Bristol Myers Squibb, Genmab, Endeavor, Immunocore, Merck, Nektar, Neon Therapeutics, Novartis, Regeneron, Replimune, Vaccinex and Xencor and contracted research through affiliated institutions from Astellas, Aulos Bio, BioAtla, Bristol Myers Squibb, Boehringer Ingelheim, Checkmate, Dragonfly, Erasca, F Star, Genentech, Immunocore, Iovance, Kite Pharma, Lyell, Merck, Nektar, Neon Therapeutics, OncoC4, Pfizer, Plexxikon, Vaccinex, Vedanta and Xencor. K.M.C. reports being a shareholder in Geneoscopy LLC and Georgiamune and has received consulting fees from Geneoscopy LLC, PACT Pharma, Tango Therapeutics, Flagship Labs 81 LLC, the Rare Cancer Research Foundation, the Jaime Leandro Foundation, Noetik and Georgiamune. W.E.C. reports no competing interests. D.A.W.: clinical trial support from Orlucent, Inc. and Blueprint Medicines. J.A.P. reports no competing interests. J.A.S. reports no competing interests. G.K.I.: institutional research grants/contracts from Pfizer, Regneron, Replmune, Bicara, Merck, Georgiamune, Obsidian, Immunocore, Iovance and Xencor; advisory boards for Pfizer, Regeneron, Replimune and Obsidian; and consultant for Pfizer. A.I.: research funding from Merck. J.H.: institutional research grants/contracts from Merck, Bristol Myers Squibb, Iovance, Lyell, Natera, Skyline and Philogen. A.S.B.: advisory board for Deciphera and research funding from Merck. N.I.K.: advisory board for Regeneron, Merck, Replimune, Immunocore, Iovance Biotherapeutics, Novartis, IO Biotech, MyCareGorithm and HUYABIO International; travel support from Castle Biosciences and Regeneron; data safety monitoring board for Incyte and AstraZeneca; scientific advisory board for T-Knife Therapeutics; study steering committee for Bristol Myers Squibb, Nektar, Regeneron and Replimune; common stock in Bellicum Pharmaceuticals and Amarin; and research funding (to institution) from Bristol Myers Squibb, Merck, Regeneron, Replimune, GlaxoSmithKline, Celgene, Novartis, IDEAYA Biosciences, Modulation Therapeutics and HUYABIO International. J. Markowitz: research support from Morphogenesis, Inc. (now TuHURA Biosciences, Inc.) and Merck. M.M.: advisory boards for Merck and Regeneron. G.N. reports no competing interests. S.K.: speakersʼ bureau for Bristol Myers Squibb. G.C.D.: advisory board for Iovance Biotherapeutics. U.S.: consultancy for Astellas, AstraZeneca, Adaptimmune, Exelixis, Gilead, Imvax, Janssen, Pfizer, Seattle Genetics and Sanofi and research funding (to institution) from Janssen, Exelixis and Astellas/Seattle Genetics. T.R. reports no competing interests. B.N.M. reports no competing interests. E.M. reports no competing interests. I.B.-C. reports no competing interests. C.R.G. reports no competing interests. I.P.G. reports no competing interests. A.V.-C. reports no competing interests. J.M.C. reports no competing interests. N.N.A.-D. reports no competing interests. S.P.P.: honoraria for advisory boards, steering committees, data safety monitoring boards or consulting from Bristol Myers Squibb, Cardinal Health, Castle Biosciences, Ideaya, Immatics, IO Biotech, Merck Sharp & Dohme, Novartis, Obsidian, OncoSec, Pfizer, Replimune, Scancell and TriSalus Life Sciences. E.S.: consulting for DE Shaw Research and advisory board for Mallinckrodt Pharmaceuticals. J. Moon reports no competing interests. M.C.W. reports no competing interests. A.R. has received honoraria for consulting for Amgen, Bristol Myers Squibb, Merck, Novartis and Roche-Genentech; is or has been a member of the scientific advisory board and holds stock in Appia, Apricity, Arcus, Compugen, CytomX, ImaginAb, ImmPact, Inspirna, Kite-Gilead, Larkspur, Lyell, Lutris, MapKure, Merus, Synthekine and Tango; and has received research funding from Agilent Technologies and from Bristol Myers Squibb through Stand Up to Cancer (SU2C) and patent royalties from Arsenal Bio.
Figures




References
-
- Carlino MS, Larkin J & Long GV Immune checkpoint inhibitors in melanoma. Lancet 398, 1002–1014 (2021). - PubMed
-
- Hughes TM et al. Desmoplastic melanoma: a review of its pathology and clinical behaviour, and of management recommendations in published guidelines. J. Eur. Acad. Dermatol. Venereol. 35, 1290–1298 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

