Targeting the MARCH5-MFN2 axis to enhance mitochondrial fusion and sensitize multiple myeloma cells to venetoclax
- PMID: 40814067
- PMCID: PMC12355794
- DOI: 10.1186/s12967-025-06942-0
Targeting the MARCH5-MFN2 axis to enhance mitochondrial fusion and sensitize multiple myeloma cells to venetoclax
Abstract
Background: Accumulating evidence suggests that mitochondrial fission and fusion events are imbalanced in cancer due to defective activity of their key regulators. In this study, we investigated the functional role of the E3 ubiquitin ligase Membrane-Associated Ring-CH-Type Finger 5 (MARCH5) in regulating cell growth, metabolic reprogramming and drug resistance in multiple myeloma (MM) through the negative regulation of the mitochondrial fusion driver mitofusin 2 (MFN2).
Methods: Cell viability and apoptosis were evaluated in MM cell lines or in co-culture with stromal cells using the CellTiter-Glo® Cell Viability Assay and Annexin V/7-AAD staining, respectively. Clonogenic potential was assessed using methylcellulose-based colony formation assays. Protein stability was determined via cycloheximide chase experiments, while protein-protein interactions by co-immunoprecipitation. Mitochondrial ultrastructure was analyzed by transmission electron microscopy. Oxygen consumption was measured using high-resolution respirometry in live cells. Transcriptomic profiling was performed using the Illumina NGS platform, and mRNA and protein levels were quantified by quantitative RT-PCR and Western blot, respectively. In vivo anti-tumor efficacy was evaluated in NOD-SCID mice subcutaneously engrafted with MM cells, using an MFN2-inducible model or following intraperitoneal administration of leflunomide. Immunohistochemistry was used to analyze tumor xenografts and mouse organs.
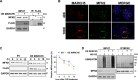
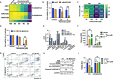
Results: Knockdown of MARCH5 led to a pronounced elongation of mitochondria accompanied by increased expression of MFN2, likely resulting from reduced MARCH5-mediated ubiquitylation. Functionally, silencing MARCH5 impaired mitochondrial oxidative phosphorylation (OXPHOS) and reduced ATP production, ultimately leading to mitochondrial dysfunction and apoptosis in MM cells. Notably, similar phenotypic and functional effects were observed following either genetic overexpression or pharmacological activation of MFN2 using leflunomide, both in vitro and in vivo in a murine xenograft model of MM. Transcriptomic profiling of MARCH5-depleted cells revealed downregulation of gene sets associated with mitochondrial electron transport chain (ETC) and ATP synthesis, pathways implicated in the development of venetoclax resistance. Consistently, both MARCH5 knockdown and MFN2 upregulation enhanced the sensitivity of MM cells to venetoclax.
Conclusion: Shifting mitochondrial dynamics toward fusion by targeting the MARCH5-MFN2 axis impairs ETC and OXPHOS, thereby sensitizing MM cells to venetoclax. These findings provide preclinical evidence for the potential therapeutic use of MFN2 inducers to enhance venetoclax responsiveness of MM patients.
Keywords: MARCH5; Mitochondrial dynamics; Multiple myeloma; Venetoclax.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Peripheral blood mononuclear cells (PBMCs) were isolated following informed consent and Institutional Review Board (University of Catanzaro, Catanzaro, Italy) approval (institutional approval: n. 266/2021). Animal experimentation was carried out at University Magna Graecia of Catanzaro under the Italian MOH authorization n. 887/2021-PR. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

