Increased autophagy activity suppresses hyperglycemia-related colorectal cancer tumorigenesis both in vitro and in vivo
- PMID: 40814362
- PMCID: PMC12344155
- DOI: 10.62347/XSRQ4118
Increased autophagy activity suppresses hyperglycemia-related colorectal cancer tumorigenesis both in vitro and in vivo
Abstract
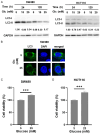
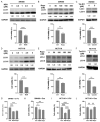
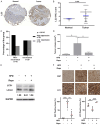
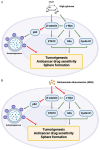
Hyperglycemia contributes to recurrence, poor survival, and drug resistance in colorectal cancer (CRC) patients. Overexpression of G9a (euchromatic histone-lysine N-methyltransferase 2, EHMT2), together with decreased autophagy activity, has been implicated in promoting CRC tumorigenesis and chemoresistance. Here, we demonstrate that high glucose (25 mM) enhances proliferation, focus formation, and migration of CRC cells, while concurrently suppressing autophagy activity. Importantly, pharmacological induction of autophagy increases CRC cell sensitivity to chemotherapeutic agents (5-fluorouracil and oxaliplatin) and attenuates high glucose-induced tumorigenic behaviors. Analysis of CRC patient specimens and data from the TCGA COAD database revealed that LC3, an autophagy marker, is elevated in tumor tissues compared to adjacent normal tissues, and that high LC3 mRNA expression correlates with poor overall survival. Furthermore, enhancing autophagy via the autophagy inducer rapamycin significantly suppressed high glucose-induced tumor formation in a CRC xenograft mouse model. In addition, we identified niclosamide (NC), a repurposed antihelminthic agent, and its derivative niclosamide ethanolamine (NEN), as potential G9a inhibitors and autophagy inducers. NEN dose-dependently suppressed high glucose-activated oncogenic signaling pathways, including β-catenin, c-Myc, STAT3, G9a, and cyclin D1, while restoring autophagy activity. Collectively, our in vitro and in vivo findings strongly support that enhancing autophagy represents a multifaceted strategy to alleviate hyperglycemia, inhibit G9a-mediated signaling, increase chemosensitivity, and suppress high glucose-driven CRC tumorigenesis.
Keywords: Autophagy; colorectal cancer; hyperglycemia; niclosamide ethanolamine.
AJCR Copyright © 2025.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Xia B, He Q, Pan Y, Gao F, Liu A, Tang Y, Chong C, Teoh AYB, Li F, He Y, Zhang C, Yuan J. Metabolic syndrome and risk of pancreatic cancer: a population-based prospective cohort study. Int J Cancer. 2020;147:3384–3393. - PubMed
-
- Yang IP, Miao ZF, Huang CW, Tsai HL, Yeh YS, Su WC, Chang TK, Chang SF, Wang JY. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther Adv Med Oncol. 2019;11:1758835919866964. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
