COVID-19-associated changes in nasal mucosa: Insights from inflammatory and epithelial cell profiling
- PMID: 40814518
- PMCID: PMC12349818
- DOI: 10.4103/jfmpc.jfmpc_185_25
COVID-19-associated changes in nasal mucosa: Insights from inflammatory and epithelial cell profiling
Abstract
Background: Coronavirus disease of 2019 (COVID-19), caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily affects nasal cavities by binding its spike proteins to angiotensin-converting enzyme 2 (ACE2) receptors. This study aimed to assess inflammatory and epithelial cell counts and determine their ratios in exfoliated nasal mucosal cells of COVID-19 suspected patients.
Methodology: This cross-sectional study was conducted at All India Institute of Medical Sciences (AIIMS), Mangalagiri, from August to October 2022, involving 75 COVID-19 suspected patients. Nasal smears were collected, stained with Papanicolaou test (PAP), and analyzed for inflammatory and epithelial cell counts. Participants were grouped as COVID-19 positive and negative based on Real Time Polymerase Chain Reaction (RT-PCR) results. Genotoxicity factors were evaluated using mean and standard deviation values.
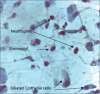
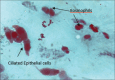
Results: In COVID-19 positive individuals, mean cell counts for neutrophils, eosinophils, lymphocytes, mast cells, macrophages, and epithelial cells were 42.08, 6.68, 4.48, 0.84, 0.12, and 45.80, respectively. In COVID-19 negative patients, the corresponding values were 38.08, 13.76, 11.12, 2.66, 0.22, and 34.16. The inflammatory ratios for neutrophils to eosinophils and neutrophils to lymphocytes in COVID-19 positive individuals were 7.30 and 14.06, respectively. SARS-CoV-2 infection resulted in elevated neutrophil and epithelial cell counts and decreased eosinophil, lymphocyte, mast cell, and macrophage count.
Conclusions: SARS-CoV-2 exhibits unique immunological alterations in nasal mucosa. The inflammatory ratios of neutrophils-to-eosinophils and neutrophils-to-lymphocytes may serve as reliable diagnostic markers for COVID-19.
Keywords: COVID-19; SARS-CoV-2; epithelial cells; inflammation; nasal mucosa.
Copyright: © 2025 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no competing conflicts of interest to declare among the authors.
Figures



Similar articles
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard [Internet] 2022. [[Last accessed on 2022 Aug 12]]. Available from: https://covid19.who.int/
-
- Nakamura T, Hasegawa-Nakamura K, Sakoda K, Matsuyama T, Noguchi K. Involvement of angiotensin II type 1 receptors in interleukin-1?-induced interleukin-6 production in human gingival fibroblasts. Eur J Oral Sci. 2011;119:345–51. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
