Non-invasive Diagnosis of Antinephrin-Associated Podocytopathy
- PMID: 40814620
- PMCID: PMC12347914
- DOI: 10.1016/j.ekir.2025.05.005
Non-invasive Diagnosis of Antinephrin-Associated Podocytopathy
Abstract
Introduction: Circulating autoantibodies against the podocyte surface protein nephrin have recently been described in patients with podocytopathies, that is, minimal change disease, primary focal segmental glomerulosclerosis, and childhood idiopathic nephrotic syndrome. Their high specificity for podocytopathies in combination with a strong correlation with disease activity hold the potential for a non-invasive diagnosis, but prospective data are lacking.
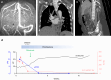
Methods: Here, we describe 3 patients with contraindications or unwillingness for a kidney biopsy, hampering a timely histological diagnosis and choice of appropriate therapy.
Results: In all patients, antinephrin autoantibodies were detected by quantitative immunoprecipitation, prompting the initiation of adequate treatment. These interventions induced a decrease in antinephrin autoantibody levels and clinical remission.
Conclusion: Our study highlights the potential of antinephrin autoantibody measurement for a noninvasive diagnosis of antinephrin-associated podocytopathy.
Keywords: antinephrin autoantibodies; minimal change disease; podocytopathy; serology.
© 2025 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
-
- Hengel F.E., Dehde S., Yilmaz A., et al. Anti-nephrin autoantibodies in steroid-resistant nephrotic syndrome may inform treatment strategy. Kidney Int. 2025;107:951. - PubMed
LinkOut - more resources
Full Text Sources

