Somatosensory Profile of Central Post Stroke Pain of Thalamic Origin: Findings of a Quantitative Sensory Testing Study
- PMID: 40814914
- PMCID: PMC12355633
- DOI: 10.1002/ejp.70104
Somatosensory Profile of Central Post Stroke Pain of Thalamic Origin: Findings of a Quantitative Sensory Testing Study
Abstract
Introduction: Central post stroke pain (CPSP) is attributed to vascular lesions of the central somatosensory system, including the thalamus.
Objective: A better characterisation of clinical findings in patients with CPSP after thalamic stroke can facilitate research and treatment of this refractory pain syndrome. We aimed to quantify somatosensory abnormalities in CPSP patients after thalamic stroke.
Methods: Sixteen patients with CPSP after thalamic stroke, 14 patients with a history of thalamic stroke without any pain (stroke control patients, SCP) and 12 healthy controls (HC) underwent detailed clinical examination, standardised quantitative sensory testing (QST) and a pain questionnaire. QST results were compared to age and sex adjusted reference data to obtain z-scores. Group comparisons were performed with one-way analysis of variance.
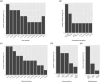
Results: Temperature perception did not differ in CPSP patients, apart from thermal sensory limen (higher in CPSP vs. HC but no difference vs. SCP). Patients with CPSP showed higher mechanical detection thresholds compared to SCP (Δ = 1.26, p = 0.017, no difference vs. HC) and they were more sensitive to mechanical pain than SCP (lower mechanical pain thresholds vs. SCP: Δ = -1.32, p = 0.014, no difference vs. HC).
Conclusion: Our results indicate somatosensory abnormalities in patients with CPSP after thalamic stroke, associated with the perception of mechanical stimuli. A short somatosensory screening including mechanical perception may contribute to an accurate diagnosis of this debilitating condition.
Significance statement: Thalamic CPSP is very rare, thus our data contribute to the clinical and sensory phenotyping of patients suffering from this debilitating condition. We did not find abnormalities in thermal measures, but only mechanical thresholds in our cohort, suggesting that not only changes in temperature perception are necessary for the development of pain after thalamic stroke. Our findings suggest that a short QST protocol including mechanical testing using von Frey filaments and pin-prick-stimulators may be useful in the diagnosis of thalamic CPSP.
Keywords: CPSP; QST; mechanical pain sensitivity; phenotyping; thalamic pain; thalamic stroke.
© 2025 The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Conflict of interest statement
Kristel Berati, Priska Zuber and Kean Schoenhoelzer do not have conflicts of interest to declare. Lukas S. Enz has received funding from the Swiss National Science Foundation (323530_171139). Katarina Alexandra Ebner received compensation for advisory board (Lundbeck), which was used for research in the University Hospital of Basel. Federico Burguet Villena received travel support form TEVA. Laura Gaetano is currently an employee of Novartis AG. Ludwig Kappos: Institutional research support: steering committee, advisory board, consultancy fees: Actelion, Bayer HealthCare, Biogen, Bristol Myers Squibb, Genzyme, Janssen, Japan Tobacco, Merck, Novartis, Roche, Sanofi, Santhera, Shionogi, and TG Therapeutics, speaker fees: Bayer HealthCare, Biogen, Merck, Novartis, Roche, and Sanofi; support of educational activities: Allergan, Bayer HealthCare, Biogen, CSL Behring, Desitin, Genzyme, Merck, Novartis, Roche, Pfizer, Sanofi, Shire, and Teva; license fees for Neurostatus products; and grants: Bayer HealthCare, Biogen, European Union, Innosuisse, Merck, Novartis, Roche, Swiss MS Society, and Swiss National Research Foundation. Stefano Magon is currently an employee of F. Hoffmann‐La Roche. Athina Papadopoulou received speaker‐fees/fees for advisory boards/consulting activities from Sanofi‐Genzyme, Eli Lilly, AbbVie, Lundbeck and TEVA and travel support from Bayer AG, Teva and Hoffmann‐La Roche. Her research was supported by the University‐ and University Hospital of Basel, the Swiss Multiple Sclerosis Society, the “Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung”, the “Freie Akademische Gesellschaft Basel” and the Swiss National Science Foundation (Project number: P300PB_174480). During the current research work, Athina Papadopoulou was supported by the Swiss National Science Foundation (PZ00P3_216468). Till Sprenger received research grants from the Swiss MS Society, Novartis Pharmaceuticals Switzerland, EFIC‐Grünenthal grant, and Swiss National Science Foundation. The current (DKD Helios Klinik Wiesbaden) or previous (University Hospital Basel) institutions of Till Sprenger have received payments for speaking or consultation from: Biogen Idec, Eli Lilly, Allergan, Actelion, ATI, Mitsubishi Pharma, Novartis, Genzyme and TEVA.
Figures

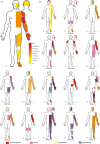
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD012574. doi: 10.1002/14651858.CD012574.pub2. Cochrane Database Syst Rev. 2022. PMID: 36477774 Free PMC article.
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2022 Jun 9;6(6):CD003870. doi: 10.1002/14651858.CD003870.pub7. Cochrane Database Syst Rev. 2022. PMID: 35679121 Free PMC article.
-
Psychological therapies for post-traumatic stress disorder and comorbid substance use disorder.Cochrane Database Syst Rev. 2016 Apr 4;4(4):CD010204. doi: 10.1002/14651858.CD010204.pub2. Cochrane Database Syst Rev. 2016. PMID: 27040448 Free PMC article.
-
Sympathetic nerve blocks for persistent pain in adults with inoperable abdominopelvic cancer.Cochrane Database Syst Rev. 2024 Jun 6;6(6):CD015229. doi: 10.1002/14651858.CD015229.pub2. Cochrane Database Syst Rev. 2024. PMID: 38842054 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

