Scavenging acrolein with 2-HDP preserves neurovascular integrity in a rat model of diabetic retinal disease
- PMID: 40815371
- PMCID: PMC12534245
- DOI: 10.1007/s00125-025-06515-2
Scavenging acrolein with 2-HDP preserves neurovascular integrity in a rat model of diabetic retinal disease
Abstract
Aims/hypothesis: Diabetic retinal disease (DRD) is characterised by progressive neurovascular unit (NVU) dysfunction, often occurring before visible microvascular damage. Our previous studies suggested that the accumulation of acrolein (ACR)-derived protein adducts on retinal Müller cells and neuronal proteins may contribute to NVU dysfunction in diabetes, although this has yet to be directly tested. In this study, we evaluated the effects of the novel ACR-scavenging drug 2-hydrazino-4,6-dimethylpyrimidine (2-HDP) on retinal NVU dysfunction in experimental diabetes and explored its potential for systemic delivery in humans.
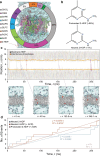
Methods: Sprague Dawley rats were divided into three groups: non-diabetic rats; streptozocin (STZ)-induced diabetic rats; and STZ-induced diabetic rats treated with 2-HDP in their drinking water throughout the duration of diabetes. Endpoint measures were taken at varying time points, ranging from 1 to 6 months post-diabetes induction. Retinal function and structure were evaluated using electroretinography (ERG) and spectral-domain optical coherence tomography (SD-OCT). Retinal vessel calibre, BP and vasopermeability (assessed by Evans Blue leakage) were also monitored. Immunohistochemistry was employed to assess retinal neurodegenerative and vasodegenerative changes, while cytokine arrays were used to investigate the effect of 2-HDP on diabetes-induced retinal inflammation. The accumulation of the ACR-protein adduct Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-Lys) in human diabetic retinas was analysed. Computational chemistry simulations were performed to predict 2-HDP's passive permeability properties and its potential for systemic delivery.
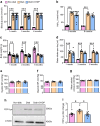
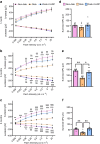
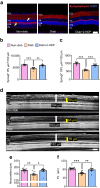
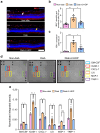
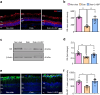
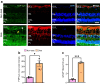
Results: 2-HDP treatment had no effect on blood glucose, body weight, water intake, HbA1c levels or BP in diabetic rats (p>0.05). However, it protected against retinal FDP-Lys accumulation (p<0.05) and neurophysiological dysfunction, preserving ERG waveforms at 3 and 6 months post-diabetes induction (p<0.05 to p<0.001 for scotopic for a-wave, b-wave and summed oscillatory potentials). SD-OCT imaging revealed that 2-HDP prevented retinal thinning at 3 months (p<0.01) and protected against synaptic dysfunction, as evidenced by preserved synaptophysin expression (p<0.01 and p<0.001 for inner and outer plexiform layers, respectively). It also prevented neurodegeneration by maintaining retinal ganglion cells, amacrine cells, bipolar cells, and photoreceptors (p<0.05 to p<0.01). In addition, 2-HDP prevented retinal arteriolar dilation (p<0.01), reduced microvascular permeability (p<0.05) and attenuated microvascular damage, as indicated by preserved pericyte numbers and reduced acellular capillary formation (p<0.05). Mechanistically, 2-HDP inhibited microglial activation (p<0.05), suppressed the upregulation of proinflammatory molecules associated with NVU dysfunction in the diabetic retina (p<0.05 to p<0.001) and preserved the expression of the Müller cell glutamate-handling proteins, glutamate aspartate transporter 1 and glutamine synthetase (p<0.05 to p<0.01). FDP-Lys accumulation was observed in post-mortem human retinas from individuals with type 2 diabetes (p<0.05), in a pattern that was similar to that in the rat model of diabetes. Molecular dynamics simulations showed that the neutral form of 2-HDP readily crosses cell membranes, with enhanced permeation in the presence of ACR, highlighting its potential for systemic delivery.
Conclusions/interpretation: 2-HDP protects against retinal NVU dysfunction in diabetic rats by reducing FDP-Lys accumulation, preserving neuroretinal function and preventing microvascular damage, independent of glycaemic control. These results, combined with evidence from human diabetic retinas and molecular dynamics simulations, support 2-HDP's potential as a promising therapeutic agent for DRD, warranting further preclinical and clinical investigation.
Keywords: Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-Lys); 2-Hydrazino-4,6-dimethylpyrimidine (2-HDP); Acrolein; Diabetic retinal disease (DRD); Electroretinography (ERG); Molecular dynamics (MD) simulations; Neurodegeneration; Neurovascular unit (NVU); Retinal inflammation; Vascular pathology.
© 2025. The Author(s).
Conflict of interest statement
Acknowledgements: We thank I. Micu and R. Delaney of the Advanced Imaging and Histology Core Technology Unit at Queen’s University Belfast for their technical assistance and use of the microscopes. We also thank the staff of the Biological Service Unit at Queen’s University Belfast for their care of the rats and assistance with animal procedures. Our sincere thanks go to the eye donors for their invaluable contribution to diabetic retinopathy research. Some of the data presented here were previously shared as a platform presentation titled ‘2-Hydrazino-4,6-dimethylpyrimidine (2-HDP) as a Novel Therapeutic for the Neurovascular Pathology of Diabetic Retinopathy’ at The Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting 2022, held in Denver, USA, from 30 April to 4 May 2022. Data availability: The data supporting the findings of this study are available from the corresponding author upon reasonable request. Funding: This study was supported by grants from Diabetes UK (18/0005791), the Health & Social Care R&D Division, Northern Ireland (STL/4748/13) and the Medical Research Council (MC_PC_15026). The funding bodies had no involvement in the study’s design, data collection, analysis, interpretation or manuscript writing. Authors’ relationships and activities: The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: TC and AS conceived the project. JA, ET, TF, CB, EB, SA, PC, CM, AR and PB performed the experiments and analysed the data. ET and MU conducted the MD simulations. TL contributed human retinal samples and advised on the design and interpretation of these experiments. TC, JA and ET wrote, edited and reviewed the paper. All authors revised the paper for intellectual content and approved the final version for publication. TC is the guarantor of the integrity of the work.
Figures


References
-
- GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health 9(2):e144–e160. 10.1016/S2214-109X(20)30489-7 - PMC - PubMed
-
- Stitt AW, Curtis TM, Chen M et al (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51:156–186. 10.1016/j.preteyeres.2015.08.001 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

