Photochemical Chain Scissions Enhance Polyethylene Glycol Biodegradability: from Probabilistic Modeling to Experimental Demonstration
- PMID: 40815374
- PMCID: PMC12392441
- DOI: 10.1021/acs.est.5c03567
Photochemical Chain Scissions Enhance Polyethylene Glycol Biodegradability: from Probabilistic Modeling to Experimental Demonstration
Abstract
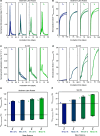
Polyethylene glycols (PEGs), a major class of water-soluble polymers (WSPs), are widely used in diverse applications, from which PEGs may be released into the environment. This work investigates the effect of PEG reaction with photochemically produced hydroxyl radicals (•OH), an important environmental oxidant, on the molecular weight (MW) distribution of PEGs and their subsequent biodegradation in soil and sediment. Monte Carlo simulations demonstrated a pronounced decrease in the PEG MW after only a few •OH-reaction-induced chain scissions on initial PEG molecules. The simulation results were validated by experimentally reacting 13C-labeled PEGs ( = 6380 ± 400 Da) with photochemically produced •OH to three extents and by analyzing the formed low MW PEG reaction products. Incubation of unreacted and •OH-reacted PEGs in both a sediment and a soil over 150 days demonstrated increasing rates and extents of PEG biodegradation into 13CO2 with increasing •OH-reaction extent and thus increasing amounts of low MW PEG products. This work underscores the importance of considering WSP MW distributions and dynamics caused by biotic or abiotic chain scission reactions when advancing a detailed understanding of WSP fate and biodegradability in natural and engineered receiving environments.
Keywords: biodegradability; chain scission; environmental fate; hydroxyl radicals; molecular weight distribution; photochemical degradation; polyethylene glycol (PEG); sediment; soil.
Figures


References
-
- Hoffmann M. M.. Polyethylene Glycol as a Green Chemical Solvent. Curr. Opin. Colloid Interface Sci. 2022;57:101537. doi: 10.1016/j.cocis.2021.101537. - DOI
-
- Gaballa S., Naguib Y., Mady F., Khaled K.. Polyethylene Glycol: Properties, Applications, and Challenges. J. Adv. Biomedical Pharm. Sci. 2023;0(0):26–36. doi: 10.21608/jabps.2023.241685.1205. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

