Electronic Patient-Reported Outcomes With Vital Sign Monitoring During Trastuzumab Deruxtecan Therapy: The PRO-DUCE Randomized Clinical Trial
- PMID: 40815510
- PMCID: PMC12357184
- DOI: 10.1001/jamanetworkopen.2025.27403
Electronic Patient-Reported Outcomes With Vital Sign Monitoring During Trastuzumab Deruxtecan Therapy: The PRO-DUCE Randomized Clinical Trial
Abstract
Importance: Patients with ERBB2 (formerly HER2 or HER2/neu)-positive metastatic breast cancer (MBC) receiving trastuzumab deruxtecan (T-DXd), a new standard of care, may experience specific adverse events affecting their quality of life (QOL). Monitoring electronic patient-reported outcomes alongside vital signs may help improve their QOL through early detection and management of these symptoms.
Objective: To assess whether symptom tracking and vital sign monitoring improve QOL compared with usual care in patients receiving T-DXd.
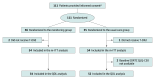
Design, setting, and participants: This multicenter randomized clinical trial conducted at 38 Japanese hospitals from March 1, 2021, to December 31, 2024, assigned patients with ERBB2-positive MBC eligible for T-DXd to either a monitoring or usual care group.
Intervention: Randomization was 1:1 for the monitoring and usual care groups. Patients in the monitoring group recorded weekly symptom reports and daily body temperature and percutaneous oxygen saturation data using a smartphone or tablet. Alerts were sent to medical staff when symptom or vital sign thresholds were exceeded.
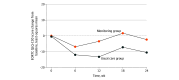
Main outcomes and measures: The primary outcome was the change from baseline in global health status scores at week 24, assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (scores range from 0 to 100, in which higher scores indicate a better quality of life and level of functioning). For symptom scales, however, higher scores indicate a worse symptom burden. Secondary outcomes included changes in functional and symptom scales, as well as survival outcomes.
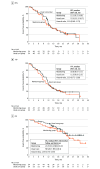
Results: Of 111 female patients enrolled (mean [SD] age, 57.1 [11.0] years), 56 (50.5%) were randomized to the monitoring group and 55 (49.5%) to the usual care group. Baseline patient characteristics and QOL scores were comparable between the 2 groups. At week 24, the mean change in the global health status score from baseline was higher in the monitoring group compared with the usual care group (mean difference, 8.0 [90% CI, 0.2-15.8]; P = .09). Improvements in functional scales were noted in the monitoring group for role (mean difference, 10.0 [95% CI, 1.1-18.9]), cognitive (6.3 [95% CI, 1.1-11.5]), and social (10.9 [95% CI, 3.9-18.0]) functioning. Fatigue scores were lower in the monitoring group (mean difference, -8.4 [95% CI, -16.1 to -0.6]), although nausea or vomiting scores were similar between the groups (mean difference, 0.5 [95% CI, -6.2 to 7.1]). No significant differences were observed in survival outcomes.
Conclusions and relevance: In this randomized clinical trial of patients with ERBB2-positive MBC receiving T-DXd, the findings suggest that electronic symptom and vital sign tracking may help maintain QOL or prevented its deterioration. While survival outcomes were unaffected, this monitoring strategy has potential to enhance patient-centered care.
Trial registration: jRCT identifier: jRCTs031200387.
Conflict of interest statement
Figures

Similar articles
-
Patient-reported outcomes with trastuzumab deruxtecan in hormone receptor-positive, HER2-low or HER2-ultralow metastatic breast cancer: results from the randomized DESTINY-Breast06 trial.ESMO Open. 2025 May;10(5):105082. doi: 10.1016/j.esmoop.2025.105082. Epub 2025 May 15. ESMO Open. 2025. PMID: 40441802 Free PMC article. Clinical Trial.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Patient-reported outcomes from DESTINY-Breast04: trastuzumab deruxtecan versus physician's choice of chemotherapy in patients with HER2-low mBC.Oncologist. 2025 May 8;30(5):oyaf048. doi: 10.1093/oncolo/oyaf048. Oncologist. 2025. PMID: 40349139 Free PMC article. Clinical Trial.
-
Specialist breast care nurses for support of women with breast cancer.Cochrane Database Syst Rev. 2021 Feb 3;2(2):CD005634. doi: 10.1002/14651858.CD005634.pub3. Cochrane Database Syst Rev. 2021. PMID: 34559420 Free PMC article.
-
Exercise interventions on health-related quality of life for people with cancer during active treatment.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. Cochrane Database Syst Rev. 2012. PMID: 22895974 Free PMC article.
References
-
- André F, Hee Park Y, Kim SB, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401(10390):1773-1785. doi: 10.1016/S0140-6736(23)00725-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

