Protocol for vibratome sectioning, immunofluorescence, and S-phase labeling of inner ear organoids
- PMID: 40815565
- PMCID: PMC12392772
- DOI: 10.1016/j.xpro.2025.104032
Protocol for vibratome sectioning, immunofluorescence, and S-phase labeling of inner ear organoids
Abstract
Inner ear organoids represent a potentially inexhaustible source of otic tissues, including sensory hair cells and supporting cells, for in vitro manipulation. Here, we present a protocol for labeling S-phase entry of cells in inner ear organoids using 5-ethynyl-2'-deoxyuridine (EdU), followed by fixation and vibratome sectioning. Nuclear EdU is then detected alongside protein markers of interest via immunofluorescence. This workflow enables the visualization of cell and tissue morphologies within developing organoids and assessment of how different manipulations affect cell proliferation. For complete details on the use and execution of this protocol, please refer to Matern et al.1.
Keywords: Cell Differentiation; Cell culture; Developmental biology; Model Organisms; Molecular/Chemical Probes; Organoids; Stem Cells; Tissue Engineering.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
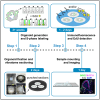
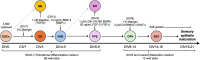
 ’ indicates pathway activation and ‘
’ indicates pathway activation and ‘ ’ indicates pathway inhibition.
’ indicates pathway inhibition.



Similar articles
-
Generation and characterization of vestibular inner ear organoids from human pluripotent stem cells.Nat Protoc. 2025 Jun 2. doi: 10.1038/s41596-025-01191-3. Online ahead of print. Nat Protoc. 2025. PMID: 40457100 Review.
-
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling.Bio Protoc. 2025 Jun 20;15(12):e5354. doi: 10.21769/BioProtoc.5354. eCollection 2025 Jun 20. Bio Protoc. 2025. PMID: 40620811 Free PMC article.
-
Protocol for differentiating human pluripotent stem cells into midbrain organoids for targeted microinjection of viruses.STAR Protoc. 2025 Jul 25;6(3):103983. doi: 10.1016/j.xpro.2025.103983. Online ahead of print. STAR Protoc. 2025. PMID: 40714558 Free PMC article.
-
Protocol for the generation and differentiation of thymic epithelial organoids from adult mouse thymus tissue.STAR Protoc. 2025 Jun 20;6(2):103883. doi: 10.1016/j.xpro.2025.103883. Epub 2025 Jun 10. STAR Protoc. 2025. PMID: 40503933 Free PMC article.
-
Fabricating mice and dementia: opening up relations in multi-species research.In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. PMID: 40690569 Free Books & Documents. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

