Training Schedule Affects Operant Responding Independent of Motivation in the Neuroligin-3 R451C Mouse Model of Autism
- PMID: 40816723
- PMCID: PMC12356647
- DOI: 10.1111/gbb.70032
Training Schedule Affects Operant Responding Independent of Motivation in the Neuroligin-3 R451C Mouse Model of Autism
Abstract
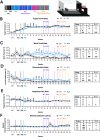
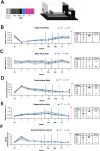
Autism affects ~1 in 100 people and arises from the interplay between rare genetic changes and the environment. Diagnosis is based on social and communication difficulties, as well as the presence of restricted and repetitive behaviours. Autism aetiology is complex. However, the social motivation hypothesis proposes that an imbalance in the salience of social over non-social stimuli contributes over time to the autism phenotype. Accordingly, motivational dysfunction in autism is widespread, and human imaging data has identified broad impairments to reward processing. The R451C mutation of the neuroligin-3 gene is one such rare genetic change. Knock-in mice harbouring this mutation (NL3) exhibit a range of autism-related phenotypes, including impaired sociability and social motivation. However, no prior report has directly probed non-social motivation. Here, we explore conflicting results from the progressive ratio (PR) and conditioned place preference tasks of non-social motivation. Initial PR results were inconsistent, suggesting reduced, unaltered, and elevated non-social motivation, respectively. Utilising several experimental designs, we probed a range of confounders likely to influence task performance. Overall, reduced PR responding by NL3s likely arose from a combination of their superior ability to withhold responding during prior training and a short PR training schedule. Meanwhile, increased PR responding by NL3s was attributable to their heightened degree of habitual responding. The NL3 mouse model therefore likely best represents autistic individuals with intact non-social motivation but altered behavioural updating. Finally, we discuss the benefits and limitations of using heterogenous experimental designs to probe behavioural phenotypes and offer some general recommendations for PR.
Keywords: R451C; atomoxetine; autism; cocaine; methylphenidate; motivation; neuroligin‐3; study design; touchscreen.
© 2025 The Author(s). Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Memantine for autism spectrum disorder.Cochrane Database Syst Rev. 2022 Aug 25;8(8):CD013845. doi: 10.1002/14651858.CD013845.pub2. Cochrane Database Syst Rev. 2022. PMID: 36006807 Free PMC article.
-
Sex differences in the social motivation of rats: Insights from social operant conditioning, behavioural economics, and video tracking.Biol Sex Differ. 2024 Jul 19;15(1):57. doi: 10.1186/s13293-024-00612-4. Biol Sex Differ. 2024. PMID: 39030614 Free PMC article.
-
Mice with an autism-associated R451C mutation in neuroligin-3 show a cautious but accurate response style in touchscreen attention tasks.Genes Brain Behav. 2022 Jan;21(1):e12757. doi: 10.1111/gbb.12757. Epub 2021 Jul 2. Genes Brain Behav. 2022. PMID: 34085373 Free PMC article.
-
Differently different?: A commentary on the emerging social cognitive neuroscience of female autism.Biol Sex Differ. 2024 Jun 13;15(1):49. doi: 10.1186/s13293-024-00621-3. Biol Sex Differ. 2024. PMID: 38872228 Free PMC article. Review.
References
-
- American Psychiatric Association , Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (American Psychiatric Association, 2022).
-
- Lai M. C., Anagnostou E., Wiznitzer M., Allison C., and Baron‐Cohen S., “Evidence‐Based Support for Autistic People Across the Lifespan: Maximising Potential, Minimising Barriers, and Optimising the Person–Environment Fit,” Lancet Neurology 19, no. 5 (2020): 434–451, 10.1016/S1474-4422(20)30034-X. - DOI - PubMed
-
- Pugsley K., Scherer S. W., Bellgrove M. A., and Hawi Z., “Environmental Exposures Associated With Elevated Risk for Autism Spectrum Disorder May Augment the Burden of Deleterious De Novo Mutations Among Probands,” Molecular Psychiatry 27, no. 1 (2022): 710–730, 10.1038/s41380-021-01142-w. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 1158460/National Health and Medical Research Council
- University of Melbourne Research Scholarship
- TDM Growth Partners and Hearts and Minds Investments
- 1111552/National Health and Medical Research Council-Australian Research Council (NHMRC-ARC) Dementia Research Development Fellowship
- Victorian Government's Operational Infrastructure Support Grant
LinkOut - more resources
Full Text Sources
Research Materials

