Protective effects of pituitary adenylate cyclase-activating peptide (PACAP) on high glucose-induced damage in human corneal epithelial cell
- PMID: 40817070
- PMCID: PMC12355837
- DOI: 10.1186/s12886-025-04309-z
Protective effects of pituitary adenylate cyclase-activating peptide (PACAP) on high glucose-induced damage in human corneal epithelial cell
Abstract
Background: Diabetic keratopathy (DK), a vision-threatening complication of diabetes mellitus, remains a significant clinical challenge. Pituitaries adenylate cyclase-activating peptide 38 (PACAP38) has been demonstrated to have neuroprotective effects. This study aimed to investigate whether PACAP38 mitigates high glucose (HG)-induced damage in human corneal epithelial cells (HCECs) and to elucidate the underlying mechanisms.
Methods: HCECs were exposed to HG to simulate diabetic injury. Cell viability, apoptosis, migration, and autophagy were evaluated using Cell Counting Kit-8 (CCK-8), flow cytometry, Transwell assays, and Western blotting, respectively.
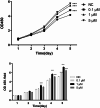
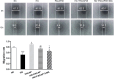
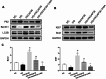
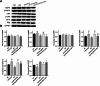
Results: Compared to the normal control (NC) group, HG significantly suppressed cell proliferation (p < 0.01), whereas PACAP38 treatment restored proliferative capacity (p < 0.01). PACAP38 enhanced cell migration, counteracting HG-induced impairment. At the molecular level, HG downregulated Ki-67 and Bcl-2 mRNA expression, while PACAP38 markedly upregulated these markers (p < 0.01). Notably, the HG impaired the autophagy in HCEC cells, while the PACAP38 significantly increased the autophagic ability. Mechanistically, PACAP38 increased the expression of p-AMPK, p-ERK, and Bcl-2 while reducing p62 (p < 0.01). Crucially, these protective effects were abolished by the autophagy inhibitor 3-MA or the AMPK inhibitor compound C (p < 0.05), confirming pathway dependency.
Conclusions: Our findings demonstrate that HG compromises HCEC proliferation and migration while promoting apoptosis. PACAP38 counteracts these detrimental effects by activating AMPK/ERK signaling, thereby enhancing cell survival and autophagy under hyperglycemic conditions. These results highlight PACAP38 as a promising therapeutic candidate for diabetic keratopathy.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12886-025-04309-z.
Keywords: Diabetic complications.; Diabetic keratopathy; High glucose; Human corneal epithelial cells; Peptides activated by pituitary adenylate cyclase.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

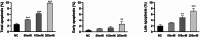
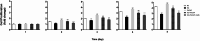

Similar articles
-
Protective effect of pituitary adenylate cyclase activating polypeptide in diabetic keratopathy.Peptides. 2023 Dec;170:171107. doi: 10.1016/j.peptides.2023.171107. Epub 2023 Sep 28. Peptides. 2023. PMID: 37775045
-
Protective effects of PACAP against high glucose-induced inflammation on air-liquid interface corneal epithelium barrier.Peptides. 2025 Jul 16;192:171432. doi: 10.1016/j.peptides.2025.171432. Online ahead of print. Peptides. 2025. PMID: 40680858
-
Development and pharmacological characterization of novel multi- calcitonin gene-related peptide and pituitary adenylate cyclase-activating peptide receptor antagonists.Headache. 2025 Jul-Aug;65(7):1064-1079. doi: 10.1111/head.14916. Epub 2025 Feb 25. Headache. 2025. PMID: 39995298 Free PMC article.
-
[Pituitary adenylate cyclase-activating polypeptide].Ann Endocrinol (Paris). 1998 Dec;59(5):364-405. Ann Endocrinol (Paris). 1998. PMID: 9949891 Review. French.
-
Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions.Pharmacol Rev. 2000 Jun;52(2):269-324. Pharmacol Rev. 2000. PMID: 10835102 Review.
References
-
- Yang Wang YY. Advances in diabetic keratopathy. Chin J Optometry Ophthalmol Visual Sci. 2020;22(12):951–5.
-
- Dan J, Zhou Q, Xie L. The research progress of relationship between advanced glycation end products and diabetic keratopathy. Chin J Ophthalmol. 2018;54(06):475–80. - PubMed
-
- Wei S, Fan J, Zhang X, Jiang Y, Zeng S, Pan X, Sheng M, Chen Y. Sirt1 attenuates diabetic keratopathy by regulating the Endoplasmic reticulum stress pathway. Life Sci. 2021;265:118789. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

