RKIP regulates bone marrow macrophage differentiation to mediate osteoclastogenesis and H-type vessel formation
- PMID: 40817102
- PMCID: PMC12356849
- DOI: 10.1038/s41467-025-62972-8
RKIP regulates bone marrow macrophage differentiation to mediate osteoclastogenesis and H-type vessel formation
Abstract
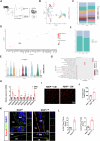
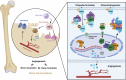
As central cells involved in osteoimmunology, bone niche macrophages possess diverse functions, and their differentiation fate regulates bone homeostasis. Elucidation of the underlying mechanism involved in macrophage differentiation is important for developing new therapeutic targets for osteoporosis. Here, we show that knocking out Raf kinase inhibitor protein (RKIP), either globally or in macrophages, results in dramatically increased bone mass in mice due to synergistic inhibition of bone resorption and promotion of bone formation. Mechanistically, RKIP knockout inhibits differentiation of macrophages into osteoclasts and promotes their differentiation towards pro-angiogenic subclusters, which enhances formation of H-type vessels. RKIP enhances osteoclastogenesis by interacting with ARHGAP to suppress CDC42 inactivation. Intranuclear RKIP suppresses angiogenic genes expression by bridging the association between HIF-1α and VHL to reduce the protein stability of HIF-1α in macrophages. Furthermore, RKIP deletion or inhibitor rescues ovariectomy (OVX)-induced bone loss in vivo. Collectively, this study provides insights into the different roles of extranuclear or intranuclear RKIP in regulating differentiation of bone niche macrophages and could inform potential therapies for bone homeostasis-related diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med.13, 791–801 (2007). - PubMed
-
- Kurihara, N., Chenu, C., Miller, M., Civin, C. & Roodman, G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology126, 2733–2741 (1990). - PubMed
MeSH terms
Substances
Grants and funding
- 82272521/National Natural Science Foundation of China (National Science Foundation of China)
- 82302732/National Natural Science Foundation of China (National Science Foundation of China)
- 82472472/National Natural Science Foundation of China (National Science Foundation of China)
- LZ23H060002/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
- LQ23H070004/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

