Design and nonviral delivery of live attenuated vaccine to prevent chronic hepatitis C virus-like infection
- PMID: 40817105
- PMCID: PMC12356917
- DOI: 10.1038/s41467-025-62813-8
Design and nonviral delivery of live attenuated vaccine to prevent chronic hepatitis C virus-like infection
Abstract
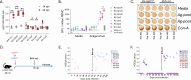
An effective vaccine for the hepatitis C virus (HCV) remains an unmet medical need. There is no animal model for assessing HCV vaccines; however, rodent hepacivirus (RHV) infection in laboratory rats recapitulates the lifelong chronic hepatotropic infection and immune evasion of HCV. Here, we designed a live-attenuated vaccine (LAV) for RHV and determined its immunogenicity and efficacy for preventing chronic infection. The LAV strains are generated by synonymous mutagenesis to increase the frequencies of naturally suppressed dinucleotides, UpA or CpG, in genomic regions that lack extensive RNA secondary structures. Rats vaccinated using LAV containing infectious virions (LAV-IV), or lipid nanoparticle-encapsulated viral RNA (LNP-vRNA) developed short-term viremia and robust T cell responses. After challenge with RHV-rn1, while all unvaccinated rats developed chronic infection, 75% and 85% of rats vaccinated with LAV-IV and LAV-vRNA cleared the infection. Clearance of RHV-rn1 was associated with expansion of memory T cells, transient rise in serum ALT, and, more importantly, enhanced protection against reinfection. In conclusion, we identified a genomic region of hepacivirus that can be synonymously mutated to attenuate its persistence, and vaccines based on these modified genomes protect against chronic hepacivirus infection, a strategy with an apparent translational path toward HCV immunization.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Houghton, M. The long and winding road leading to the identification of the hepatitis C virus. J. Hepatol.51, 939–948 (2009). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI151175/AI/NIAID NIH HHS/United States
- R01 AI183877/AI/NIAID NIH HHS/United States
- R21 AI171928/AI/NIAID NIH HHS/United States
- AI171928, AI151175, AI185926, AI183877/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI185926/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

