Androgens drive SLC1A5-dependent metabolic reprogramming in polycystic ovary syndrome
- PMID: 40817119
- PMCID: PMC12356975
- DOI: 10.1038/s41467-025-62951-z
Androgens drive SLC1A5-dependent metabolic reprogramming in polycystic ovary syndrome
Abstract
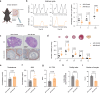
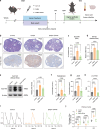
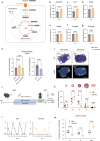
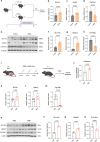
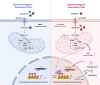
Polycystic ovary syndrome is the primary cause of female infertility. Growing evidence suggests that dysregulation of amino acid metabolism plays a significant role in the onset and progression. However, the underlying mechanism remains unclear. In this study, we conduct targeted metabolite profiling of human follicular fluid and granulosa cells. A significant increase in glutamine uptake is observed in patients with hyperandrogenic polycystic ovary syndrome, mediated by the upregulation of SLC1A5, a specific glutamine transporter. We find that androgen excess primarily activates SLC1A5 expression. Furthermore, SLC1A5 overexpression in female mice induces polycystic ovary syndrome-like phenotypes, including hyperandrogenism and abnormal follicle development. Additionally, the pharmacological blockade of SLC1A5 provides reproductive benefits to mice exhibiting polycystic ovary syndrome-like symptoms. Mechanistically, we show that elevated flux of Gln-derived α-ketoglutarate enhances HDAC5 expression and suppresses acetylation on histone 3 lysine residue 14 and lysine residue 56. The reduction in acetylation level is associated with the downregulation of several genes related to folliculogenesis, including CYP19A1, thereby exacerbating androgenic homeostasis imbalance. These findings indicate that androgen-induced aberrant glutamine uptake via SLC1A5 is crucial for the development and progression of polycystic ovary syndrome, suggesting pharmacological blockade of SLC1A5 as a potential therapeutic strategy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Boyle, J. A. & Teede, H. J. Refining diagnostic features in PCOS to optimize health outcomes. Nat. Rev. Endocrinol.12, 630–631 (2016). - PubMed
-
- Joham, A. E. et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol.10, 668–680 (2022). - PubMed
-
- Escobar-Morreale, H. F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol.14, 270–284 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

