Clinical use of vedolizumab subcutaneous formulation in inflammatory bowel diseases: a review of real-world evidence
- PMID: 40817170
- PMCID: PMC12356757
- DOI: 10.1007/s00384-025-04966-y
Clinical use of vedolizumab subcutaneous formulation in inflammatory bowel diseases: a review of real-world evidence
Abstract
Introduction: Vedolizumab is an advanced therapy indicated for the treatment of moderately to severely active Crohn's disease (CD) or ulcerative colitis (UC). After induction with vedolizumab intravenous (IV), maintenance is with vedolizumab 300 mg IV every 8 weeks, or patients can transition to vedolizumab 108 mg subcutaneous (SC) every 2 weeks.
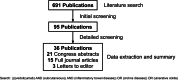
Methods: This was a literature review of the PubMed and Embase databases up to March 2024 to identify publications describing clinical practice experiences on the transition from vedolizumab IV to SC.
Results: In total, 36 eligible publications were identified, comprising 4105 UC and CD patients treated with vedolizumab: 2718 with vedolizumab SC. Across studies, there was no loss of effectiveness after transition from vedolizumab IV to SC based on disease activity scores or biomarkers (C-reactive protein and fecal calprotectin). Higher vedolizumab trough levels were observed consistently with vedolizumab SC versus IV. Treatment persistence with vedolizumab SC ranged from 89.0% to 95.5% after 3 - 6 months and approximately 81% to 89% at 12 months. A willingness to transition to the SC formulation was reported by 47% to 59% of patients, due to time savings and fewer hospital visits. Patients who transitioned to vedolizumab SC showed a high level of satisfaction (75% - 95%) with the transition. The most frequently reported adverse events with vedolizumab SC were injection site reactions, with frequencies of 2.9% to 18.5%.
Conclusions: Clinical practice experience in inflammatory bowel disease patients who transitioned from vedolizumab IV to SC showed no change in effectiveness or safety outcomes, and a high level of patient satisfaction overall.
Keywords: Crohn’s disease; Intravenous; Real-world evidence; Subcutaneous; Ulcerative colitis; Vedolizumab.
© 2025. Takeda Development Center Americas, Inc.
Conflict of interest statement
Declarations. Competing interests: KBR is a consultant and has served as an advisory board member for Bristol Myers Squibb, Pfizer, and Iterative Scopes. CA, PK, and AMW are employees of and hold stock/options in Takeda. AA is a consultant and has served as an advisory board member for AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, and Takeda, and has received speaker’s fees from AbbVie, Bristol Myers Squibb, Janssen, Pfizer, and Takeda.
References
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L (2017) Crohn’s disease. Lancet 389:1741–1755 - PubMed
-
- Turner D, Ricciuto A, Lewis A et al (2021) STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160:1570–1583 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials