Dual-vector rAAVrh8 gene therapy for GM2 gangliosidosis: a phase 1/2 trial
- PMID: 40817303
- PMCID: PMC12443631
- DOI: 10.1038/s41591-025-03822-4
Dual-vector rAAVrh8 gene therapy for GM2 gangliosidosis: a phase 1/2 trial
Abstract
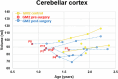
The dual rAAVrh8-HEXA and rAAVrh8-HEXB vector can restore central nervous system hexosaminidase (Hex) enzyme activity, decrease GM2 levels in cerebrospinal fluid and rescue phenotypic consequences of GM2 gangliosidosis, Tay-Sachs and Sandhoff diseases in animal models following simultaneous bi-thalamic (BiT) injections. Following up on an n = 2 expanded access trial, we initiated a phase 1/2, single-dose, dose-escalation of combined BiT, intra-cisterna magna and intrathecal infusion in children with Tay-Sachs and Sandhoff diseases (six infantile, three juvenile). The BiT injection volume and vector dose were doubled between four cohorts, with the lowest dose matching the earlier expanded access trial. Cerebrospinal fluid HexA enzyme activity, serum total Hex activity and GM2 levels showed a dose-dependent biochemical correction of the disease. Serum Hex activity surpassed 40 nmol h-1 ml-1, two times the lower limit of normal, and neuroimaging demonstrated increased fiber tracts. Correction was greatest at 12 weeks, but in decline by 24 weeks postdosing. Infantile patients experienced global clinical stabilization and prolonged oral feeding without aspiration until 3-3.5 years. Seizures had a later onset, were less frequent, less severe and more responsive to anti-convulsant medication. Adverse events were rare in infantile patients, but worsening dystonia was observed in juvenile patients, who were excluded from ongoing enrollment. ClinicalTrials.gov registration: NCT04669535 and NCT06614569 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.S.-E. may be entitled to receive licensing revenue from the patents covering the technologies used in this study. The remaining authors declare no competing interests.
Figures









References
-
- Day, J. W. et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol.20, 284–293 (2021). - PubMed
-
- Hwu, W.-L. et al. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci. Transl. Med.4, 134ra61 (2012). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

