Analgesia, Sedation, and Neuromuscular Blockade in Pediatric Severe Traumatic Brain Injury: Secondary Analysis of the "Approaches and Decisions in Acute Pediatric TBI Trial" (ADAPT)
- PMID: 40817360
- PMCID: PMC12459016
- DOI: 10.1007/s12028-025-02336-8
Analgesia, Sedation, and Neuromuscular Blockade in Pediatric Severe Traumatic Brain Injury: Secondary Analysis of the "Approaches and Decisions in Acute Pediatric TBI Trial" (ADAPT)
Abstract
Background: Sedative, analgesia, and neuromuscular blocking (NMB) medications may be necessary in the acute management of pediatric severe traumatic brain injury (sTBI), yet limited data exist to guide their use. We aimed to describe the use of continuous infusions of these medications in children with sTBI, to determine temporal trends during the first week of management, and to evaluate associations with in-hospital mortality.
Methods: We conducted a secondary analysis of the Approaches and Decisions in Acute Pediatric Traumatic Brain Injury Trial (NCT04077411, 2014-2017), a prospective multicenter observational study of patients < 18 years old with sTBI (Glasgow Coma Scale ≤ 8) who underwent intracranial pressure monitoring. Continuous analgesic, sedative, and NMB medication infusions administered in the first 7 days after sTBI were analyzed.
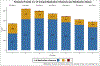
Results: Data from 929 patients were analyzed with a median Glasgow Coma Scale of 6 (interquartile range 3-7), 14% hospital mortality. In the 7 days after intracranial pressure monitor placement, 866 (93%) patients received an opioid infusion, with 659 (71%) patients having received fentanyl. A total of 679 (73%) patients received benzodiazepine: 671 (72%) patients received midazolam. A total of 362 (39%) patients received NMB, with the most common being vecuronium, administered to 141 (15%) patients. Propofol was administered to 264 (28%) patients, alpha-2 agonist to 263 (28%) patients, and ketamine to 4 (0.43%) patients. The median number of infusions per patient was 2 (interquartile range 1-2), with the highest number on intensive care unit day 2. Morphine and dexmedetomidine infusions were used more often in survivors than nonsurvivors (33 vs. 16%, and 30 vs. 9%, respectively, p < 0.001).
Conclusions: Fentanyl and midazolam were the most common analgesic and sedative continuous infusions during acute pediatric sTBI management. Propofol and dexmedetomidine were used less frequently. Opioid (specifically morphine) and dexmedetomidine infusions were associated with survival. Larger studies are needed to determine the safest and most effective analgesia, sedation, and NMB medication strategy for children with sTBI.
Keywords: Analgesia; Brain injuries (traumatic); Hypnotics and sedatives; Intracranial pressure; Neuromuscular blockade.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: Each author has completed an International Committee of Medical Journal Editors Conflict of Interest form, included with this submission. We report no significant conflicts of interest. Dr. Buttram served as a site Principal Investigator for the original data collection of the Approaches and Decisions for Acute Pediatric TBI Trial. Ethical Approval: The institutional review boards of Vanderbilt University Medical Center and Phoenix Children’s Hospital determined our investigation as nonhuman study participants research not requiring informed consent.
Figures


References
-
- Fink EL, Kochanek PM, Tasker RC, et al. International Survey of Critically Ill Children With Acute Neurologic Insults: The Prevalence of Acute Critical Neurological Disease in Children: A Global Epidemiological Assessment Study*. Pediatr Crit Care Med. 2017;18(4):330. doi: 10.1097/PCC.0000000000001093 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. National Center for Health Statistics: Mortality Data on CDC WONDER. Accessed August 15, 2024. https://wonder.cdc.gov/Deaths-by-Underlying-Cause.html
-
- Data TBI and Statistics | Concussion | Traumatic Brain Injury | CDC Injury Center. Accessed February 28, 2021. https://www.cdc.gov/traumaticbraininjury/data/
Grants and funding
LinkOut - more resources
Full Text Sources

