High-dose ascorbic acid selectively induces pyroptosis in LKB1-deficient lung cancer and sensitizes immunotherapy
- PMID: 40818456
- PMCID: PMC12490232
- DOI: 10.1016/j.xcrm.2025.102291
High-dose ascorbic acid selectively induces pyroptosis in LKB1-deficient lung cancer and sensitizes immunotherapy
Abstract
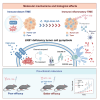
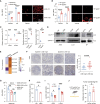
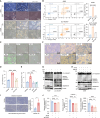
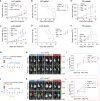
Liver kinase B1 (LKB1)-deficient non-small cell lung cancers (NSCLCs) exhibit primary resistance to immune checkpoint inhibitors (ICIs). The redox imbalance inherent in these tumors may represent a potential therapeutic vulnerability. High-dose ascorbic acid (AA) could induce cell redox imbalance. Here, we uncover that LKB1 deficiency upregulates the transporter GLUT1, which enables the accumulation of AA, thereby exacerbating redox imbalance in NSCLC cells. This triggers pyroptosis in LKB1-deficient NSCLC cells via the H2O2/reactive oxygen species (ROS)-caspase-3-gasdermin-E (GSDME) axis. In pre-clinical models, high-dose AA reverses ICI resistance and remodels the immune microenvironment, characterized by T cell factor 1 (TCF1)+CD8+ T cell (progenitor-exhausted CD8+ T cell [Tpex]) infiltration. Pyroptosis-driven immunogenic cell death (ICD) promotes cross-presenting dendritic cell (DC) maturation, which drives Tpex proliferation. Crucially, in Batf3-/- mice lacking functional CD103+ DC populations, both Tpex expansion and therapeutic benefits are abrogated, confirming DC dependence. In addition, GSDME is validated as a gatekeeper of pyroptosis-driven antitumor immunity. This work provides a rationale for clinical trials combining ICI with high-dose AA.
Keywords: GSDME; LKB1-deficient lung cancer; Tpex; caspase-3; dendritic cells; high-dose ascorbic acid; immunotherapy resistance; pyroptosis; reactive oxygen species.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Skoulidis F., Goldberg M.E., Greenawalt D.M., Hellmann M.D., Awad M.M., Gainor J.F., Schrock A.B., Hartmaier R.J., Trabucco S.E., Gay L., et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. - DOI - PMC - PubMed
-
- Calles A., Sholl L.M., Rodig S.J., Pelton A.K., Hornick J.L., Butaney M., Lydon C., Dahlberg S.E., Oxnard G.R., Jackman D.M., Jänne P.A. Immunohistochemical Loss of LKB1 Is a Biomarker for More Aggressive Biology in KRAS-Mutant Lung Adenocarcinoma. Clin. Cancer Res. 2015;21:2851–2860. doi: 10.1158/1078-0432.CCR-14-3112. - DOI - PubMed
-
- Rahim M.K., Okholm T.L.H., Jones K.B., McCarthy E.E., Liu C.C., Yee J.L., Tamaki S.J., Marquez D.M., Tenvooren I., Wai K., et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127–1143.e18. doi: 10.1016/j.cell.2023.02.021. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

