Regulatory T cell therapy promotes TGF-β and IL-6-dependent pro-inflammatory Th17 cell generation by reducing IL-2
- PMID: 40818959
- PMCID: PMC12357911
- DOI: 10.1038/s41467-025-62628-7
Regulatory T cell therapy promotes TGF-β and IL-6-dependent pro-inflammatory Th17 cell generation by reducing IL-2
Abstract
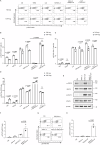
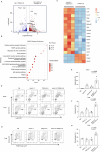
CD4+Foxp3+ regulatory T cells are essential for maintaining immune tolerance and preventing excessive inflammation, making them promising candidates for treating autoimmunity and GvHD. However, the translation of regulatory T cell therapy into clinical practice poses substantial challenges. Here, we show that adoptive regulatory T cell therapy increases IL-6 and TGF-β-dependent pathogenic Th17 cell differentiation in murine models of inflammatory bowel disease and experimental autoimmune encephalomyelitis. Regulatory T cells increase the p-stat3/p-stat5 ratio in effector T cells by suppressing IL-2 secretion and competitively consuming IL-2, thereby promoting Th17 cell differentiation. Notably, IL-2 signaling deficiency not only promotes a Th17 cell-associated transcriptional program, but also enhances the pro-inflammatory properties of Th17 cells. Strikingly, therapeutic blockade of IL-6/STAT3 signaling pathway can reverse pathogenic Th17 cell differentiation and enhance the therapeutic effect of regulatory T cell therapy. Thus, our findings could potentially advance the clinical research progress of adoptive regulatory T cell therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol.155, 1151–1164 (1995). - PubMed
-
- Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol.4, 330–336 (2003). - PubMed
-
- Sakaguchi, S., Wing, K., Onishi, Y., Prieto-Martin, P. & Yamaguchi, T. Regulatory T cells: how do they suppress immune responses?. Int. Immunol.21, 1105–1111 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

