Genkwanin glycosides are major active compounds in Phaleria nisidai extract mediating improved glucose homeostasis by stimulating glucose uptake into adipose tissues
- PMID: 40818962
- PMCID: PMC12357923
- DOI: 10.1038/s41467-025-62689-8
Genkwanin glycosides are major active compounds in Phaleria nisidai extract mediating improved glucose homeostasis by stimulating glucose uptake into adipose tissues
Abstract
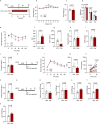
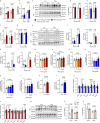
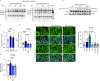
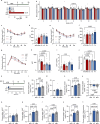
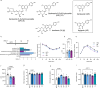
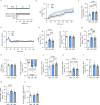
Natural remedies are used as standalone treatments or complementary to modern medicine to control type 2 diabetes. In Palau, the traditional leaf decoction of Phaleria nisidai (PNe) is selected to treat hyperglycemia and its efficacy has been supported by a small clinical trial. As part of a reverse pharmacology approach, we here investigated the anti-diabetic potential of PNe and its bioactive compounds to alleviate insulin resistance in diet-induced obese, male mice. Dietary supplementation with PNe improves insulin sensitivity and promotes glucose uptake into adipose depots. In vitro, PNe triggers glucose disposal into murine and human adipocytes by upregulating Glut1 expression through PKC-ERK1/2 signaling. To identify active constituents in PNe, we conducted bioactivity-guided fractionations and deciphered genkwanin flavone glycosides as bioactive principles. Moreover, we demonstrate that the aglycone genkwanin (GE) improves insulin resistance to a comparable extent to the anti-diabetic drug, metformin. Our findings present GE as promising glucoregulatory phytochemical that facilitates glucose uptake into adipocytes, thereby reducing systemic glucose load and enhancing insulin sensitivity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature444, 840–846 (2006). - PubMed
-
- Tremblay, F., Lavigne, C., Jacques, H. & Marette, A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes50, 1901–1910 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

