Alpha-synuclein interacts with regulators of ATP homeostasis in mitochondria
- PMID: 40819080
- PMCID: PMC12357861
- DOI: 10.1038/s41467-025-62895-4
Alpha-synuclein interacts with regulators of ATP homeostasis in mitochondria
Abstract
Mitochondrial dysfunction and accumulation of α-synuclein aggregates are hallmarks of the neurodegenerative Parkinson's disease and may be interconnected. To investigate the interplay between α-synuclein and brain mitochondria at near atomic structural level, we apply NMR and identify α-synuclein protein interactors using limited proteolysis-coupled mass spectrometry (LiP-MS). Several of the proteins identified are related to ATP synthesis and homeostasis and include subunits of ATP synthase and the adenylate kinase AK2. Furthermore, our data suggest that α-synuclein interacts with the Parkinson's disease-related protein DJ1. NMR analysis demonstrates that both AK2 and DJ1 bind to the C-terminus and other segments of α-synuclein. Using a functional assay for AK2, we show that monomeric α-synuclein has an activating effect, whereas C-terminally truncated α-synuclein and α-synuclein in an amyloid fibrillar state have no significant effect on AK2 activity. Our results suggest that α-synuclein modulates ATP homeostasis in a manner dependent on its conformation and its C-terminal acidic segment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Paola Picotti is a scientific advisor for the company Biognosys AG (Zurich, Switzerland) and an inventor of a patent licensed by Biognosys AG that covers the LiP-MS method used in this manuscript. All other authors declare no competing interests.
Figures
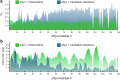

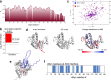

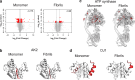
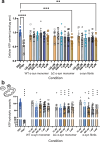
References
-
- de Rijk, M. C. et al. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology48, 1277–1281 (1997). - PubMed
-
- Balestrino, R. & Schapira, A. H. V. Parkinson disease. Eur. J. Neurol.27, 27–42 (2020). - PubMed
-
- von Campenhausen, S. et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur. Neuropsychopharmacol.15, 473–490 (2005). - PubMed
-
- McCann, H., Stevens, C. H., Cartwright, H. & Halliday, G. M. Alpha-synucleinopathy phenotypes. Parkinsonism Relat. Disord.20, S62–67 (2014). - PubMed
-
- Theillet, F. X. et al. Structural disorder of monomeric alpha-synuclein persists in mammalian cells. Nature530, 45–50 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

