Essential minerals in colostrum of preterm and full-term Ghanaian mothers and related maternal factors
- PMID: 40819102
- PMCID: PMC12357879
- DOI: 10.1038/s41598-025-15815-x
Essential minerals in colostrum of preterm and full-term Ghanaian mothers and related maternal factors
Abstract
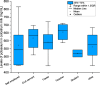
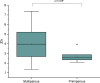
Mineral elements in colostrum play crucial roles in neonatal growth and development. This study assessed the concentrations of ten essential mineral elements in the colostrum of moderately preterm and full-term mothers in Ghana, and examined their associations with maternal characteristics. A total of 47 mothers provided single 12 mL colostrum samples collected between postpartum days 1 to 4 using standardized, aseptic manual expression procedures. The samples were digested and analyzed using ICP-OES. Statistical analyses included tests for normality, Spearman correlation, and group comparisons using Kruskal-Wallis and Mann-Whitney U tests. The concentrations ranged from 0.1 ± 0.0 mg/L (Se) to 602.6 ± 77.6 mg/L (K). Significant positive correlations were observed among several mineral pairs. Notably, potassium levels were significantly associated with maternal employment status (p = 0.047), and zinc levels were significantly related to maternal parity (p = 0.028). Although differences between preterm and full-term samples were not statistically significant, preterm colostrum showed trends of higher zinc and potassium concentrations. Compared to global data, the Ghanaian samples exhibited relatively elevated levels of phosphorus, sodium, and selenium. These findings provide foundational data on early breast milk composition in Ghana and highlight maternal and gestational factors that may influence neonatal mineral intake.
Keywords: Breast milk; Breastfeeding; Colostrum; Essential mineral elements; Lactation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Ethical approval for the study was given by the University of Health and Allied Sciences Research Ethics Committee, Protocol Number: [UHAS-REC A.9 [6] 19–20]. Informed consent: Written informed consent was obtained from all participants before they participated in the study.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical