Multi-laboratory comparisons of manual patch clamp hERG data generated using standardized protocols and following ICH S7B Q&A 2.1 best practices
- PMID: 40819150
- PMCID: PMC12357877
- DOI: 10.1038/s41598-025-15761-8
Multi-laboratory comparisons of manual patch clamp hERG data generated using standardized protocols and following ICH S7B Q&A 2.1 best practices
Abstract
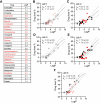
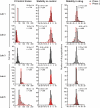
Acute block of hERG channels is the most common mechanism underlying drug-induced QTC prolongation and potentially fatal Torsade de Pointes arrhythmia. Updates to ICH E14 Q&As now allow for using negative nonclinical data, including hERG, to support QTC risk assessment in late-stage clinical development. To interpret the hERG results, understanding hERG assay reproducibility or hERG data variability is pivotal. Protocol and best practice recommendations have been provided with the goal of minimizing lab-to-lab data differences, but the impact remains unclear. To fill this knowledge gap, hERG data from a HESI-coordinated multi-laboratory study were leveraged. Using standardized protocol and following best practices for patch clamp studies, five laboratories tested 28 drugs using the manual patch clamp technique. Systematic differences in block potencies were observed for data generated by one laboratory for the first 21 drugs, and these differences disappeared for the last seven drugs. Exposure, pharmacological sensitivity of the cell lines, and cell/data qualities were ruled out as the factors underlying systematic differences. All laboratories retested two drugs and obtained results within 1.6X of the initial testings, except for another laboratory that obtained data for one drug that differed from its initial testing by 7.6X. Descriptive statistics and meta-analysis were applied to the dataset to estimate what the distribution in hERG block potencies would be if a laboratory were to test the same drug repeatedly. This measure, or hERG data variability, was ~ 5X. Based on these results, hERG block potency values within 5X of each other should not be considered different, since these values are within the natural data distribution of the hERG assay; laboratory-specific safety margin threshold may be required to account for systematic data differences.
Keywords: Assay reproducibility; Cardiac safety; Concentration-inhibition; Delayed ventricular repolarization; Experimental uncertainty; Safety margin.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Legacy hERG study data in contemporary integrated risk assessments - mitigating the risk of protocol and positive control article differences.J Pharmacol Toxicol Methods. 2025 Jul 17;135:108387. doi: 10.1016/j.vascn.2025.108387. Online ahead of print. J Pharmacol Toxicol Methods. 2025. PMID: 40683363
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Opioids-induced inhibition of HERG ion channels and sudden cardiac death, a systematic review of current literature.Trends Cardiovasc Med. 2024 Jul;34(5):279-285. doi: 10.1016/j.tcm.2023.03.006. Epub 2023 Apr 2. Trends Cardiovasc Med. 2024. PMID: 37015297
References
-
- Anonymous ICH S7B: the non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. The international Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH). (2005). https://database.ich.org/sites/default/files/S7B_Guideline.pdf.
-
- Anonymous & ICH E14. : The clinical evaluation of qt/qtc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. The international Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH). (2005). https://database.ich.org/sites/default/files/E14_Guideline.pdf.
-
- Anonymous & ICH E14/S7B IWG. : Questions and answers: clinical and nonclinical evaluation of qt/qtc interval prolongation and proarrhythmic potential.the international Council for harmonisation of technical requirements for pharmaceuticals for human use (ICH). (2022). https://database.ich.org/sites/default/files/ICH_E14-S7B_QAs_Step2_2020_....
-
- Kirsch, G. E. et al. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J. Pharmacol. Toxicol. Methods. 50, 93–101. 10.1016/j.vascn.2004.06.003 (2004). - PubMed
-
- Guo, J., Zhan, S., Lees-Miller, J. P., Teng, G. & Duff, H. J. Exaggerated block of hERG (KCNH2) and prolongation of action potential duration by erythromycin at temperatures between 37 degrees C and 42 degrees C. Heart Rhythm. 2, 860–866. 10.1016/j.hrthm.2005.04.029 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

