Albumin-based nanoparticles encapsulating SN-38 demonstrate superior antitumor efficacy compared to irinotecan
- PMID: 40819311
- PMCID: PMC12360046
- DOI: 10.1080/10717544.2025.2545519
Albumin-based nanoparticles encapsulating SN-38 demonstrate superior antitumor efficacy compared to irinotecan
Abstract
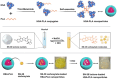
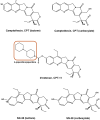
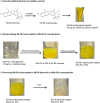
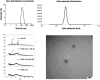
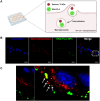
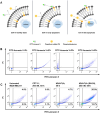
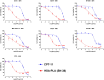
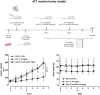
Efficient formulation of SN-38 for broad-spectrum chemotherapy remains an unmet medical need. The limited solubility of SN-38 in both aqueous and organic solvents poses a major challenge for formulation development. As a result, the predominant strategy, polymer-SN-38 drug conjugates, often involves complex synthetic procedures and low drug loading (1-5% w/w). Such limitations hinder their large-scale production and clinical translation. In this study, we developed an encapsulation strategy that utilizes the reversible lactone-carboxylate equilibrium of SN-38 to simplify the formulation process and achieve enhanced drug loading. The major issue of SN-38 solubility in organic solvents was effectively addressed by sodium hydroxide (NaOH)-induced conversion of the lactone to the carboxylate form. We have demonstrated that SN-38 carboxylate, once encapsulated within human serum albumin-polylactic acid (HSA-PLA) nanoparticles, retains its reversibility and can be converted back to the active lactone form simply by the addition of hydrochloric acid (HCl). The drug loading capacity of SN-38 in the HSA-PLA nanoparticles was increased to 19% w/w. In vitro cytotoxicity assays confirmed that HSA-PLA (SN-38) nanoparticles exhibited significantly lower IC50 values (0.5-194 nM) across multiple cancer cell lines compared to the clinical standard, irinotecan (CPT-11), indicating superior potency under physiological conditions. In vivo studies in 4T1 and MDA-MB-231 tumor-bearing mice further validated the enhanced therapeutic efficacy of this formulation. Overall, this study presents a promising alternative strategy for SN-38 delivery via encapsulation rather than polymer-drug conjugation, significantly simplifying the formulation process and enhancing the translational potential of SN-38 for broad chemotherapeutic applications.
Keywords: HSA–PLA; Human serum albumin; Protein drug delivery system; SN-38 loaded nanoparticles; lactone–carboxylate equilibrium.
Conflict of interest statement
Guojun Xiong, Andreas G. Schätzlein, and Ijeoma F. Uchegbu are inventors of a PCT patent (WO2025083423) filed by the UCLB. This work has no conflict of interest with Nanomerics Ltd.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Amphiphilic albumin-based nanoparticles designed for the efficient delivery of taxanes.Int J Pharm. 2025 Sep 15;682:125965. doi: 10.1016/j.ijpharm.2025.125965. Epub 2025 Jul 13. Int J Pharm. 2025. PMID: 40664342
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
GSH/pH-Responsive Chitosan-PLA Hybrid Nanosystems for Targeted Ledipasvir Delivery to HepG2 Cells: Controlled Release, Improved Selectivity, DNA Interaction, Electrochemical and Stopped-Flow Kinetics Analyses.Int J Mol Sci. 2025 Jun 24;26(13):6070. doi: 10.3390/ijms26136070. Int J Mol Sci. 2025. PMID: 40649846 Free PMC article.
-
Multifunctional delivery strategies and nanoplatforms of SN-38 in cancer therapeutics.J Control Release. 2025 Aug 10;384:113937. doi: 10.1016/j.jconrel.2025.113937. Epub 2025 Jun 7. J Control Release. 2025. PMID: 40490199 Review.
References
-
- Jiang M, Li W, Liang J, et al. 2024. Developing a palladium(II) agent to overcome multidrug resistance and metastasis of liver tumor by targeted multiacting on tumor cell, inactivating cancer-associated fibroblast and activating immune response. J Med Chem. 67(18):16296–16310. doi: 10.1021/acs.jmedchem.4c01175. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
