NADPH oxidase 1/4 dual inhibition impairs transforming growth factor-beta protumorigenic effects in cholangiocarcinoma cancer-associated fibroblasts
- PMID: 40820091
- PMCID: PMC12358586
- DOI: 10.1038/s41392-025-02347-z
NADPH oxidase 1/4 dual inhibition impairs transforming growth factor-beta protumorigenic effects in cholangiocarcinoma cancer-associated fibroblasts
Abstract
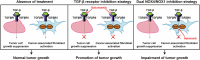
Transforming growth factor beta (TGF-β) signalling has become an attractive therapeutic target due to its pro-tumorigenic actions on epithelial cells and its immunosuppressive effects in the tumour microenvironment. In intrahepatic cholangiocarcinoma (iCCA), a highly aggressive malignancy of the biliary tract with poor prognosis, the latest clinical trials using TGF-β inhibitors have failed indicating that the specific actions carried out by TGF-β in iCCA are yet not well delineated. Here, we show that TGF-β signalling is highly active in iCCA and exerts a prominent suppressor effect on tumour cell lines and organoids established from iCCA metastases biopsies, that relies on a functional canonical SMAD2/3/4 signalling. Thus, TGF-β inhibitors promote, instead of inhibiting, tumour cell growth. In this context, a promising strategy is to target intracellular proteins downstream the TGF-β receptors accounting only for TGF-β pro-tumorigenic actions. NADPH oxidase 4 (NOX4), a downstream mediator of the TGF-β signalling pathway, is strictly expressed in cancer-associated fibroblasts (CAF) of iCCA and acts in concert with NOX1 to regulate CAF functions. Use of a dual NOX4/NOX1 inhibitor impaired CAF actions and reduced tumour growth in vitro and in two different in vivo iCCA experimental models. Collectively, our findings reveal an actionable way to specifically target TGF-β pro-tumorigenic actions in CAF from iCCA without undesirable side effects on tumour cells, suggesting a potentially bright future for dual NOX4/NOX1 inhibitors in the clinics, alone or in combination with other therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.V.T. received research grants from AstraZeneca, Lilly Oncology, Servier, Alentis, and Incyte, which are irrelevant to the current study. Personal fee from AstraZeneca and Incyte. Remaining authors have no conflicts of interest to declare related to this manuscript.
Figures
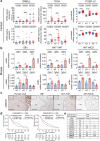



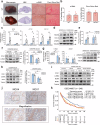

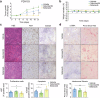
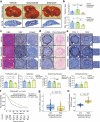
References
-
- Banales, J. M. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol.13, 261–280 (2016). - PubMed
-
- Oh, D. Y. et al. Plain language summary of the TOPAZ-1 study: durvalumab and chemotherapy for advanced biliary tract cancer. Future Oncol.19, 2277–2289 (2023). - PubMed
-
- Rimini, M. et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: An early exploratory analysis of real-world data. Liver Int43, 1803–1812 (2023). - PubMed
-
- Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet401, 1853–1865 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

