PcMYBs responded to 6-BA to regulate PcCKXs to promote germination of primary rhizome buds of Polygonatum cyrtonema Hua
- PMID: 40820132
- PMCID: PMC12359928
- DOI: 10.1186/s12870-025-06827-w
PcMYBs responded to 6-BA to regulate PcCKXs to promote germination of primary rhizome buds of Polygonatum cyrtonema Hua
Abstract
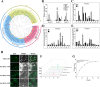
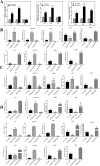
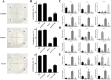
Polygonatum cyrtonema Hua is valued both as a precious traditional Chinese medicinal herb and as a prime example of a plant that bridges medicinal and culinary applications. Renowned for its significant medicinal and edible qualities, this botanical exemplifies a unique convergence of therapeutic and nutritional benefits. However, the primary rhizome of Polygonatum cyrtonema Hua development is difficult to germinate into seedlings in the same year. The germination of primary rhizome buds of P. cyrtonema can be promoted by treatment with exogenous hormone 6-BA, but the related regulatory mechanism is not clear. In this study, we found that the cytokinin oxidase (CKX) plays a key role in the germination of primary rhizome buds of P. cyrtonema. PcCKX1,2,3 promoted the expression of dormancy positively regulated genes, and repressed the expression of dormancy negatively regulated genes, which in turn inhibited Arabidopsis seed germination, and PcCKX2 was the major gene. PcCKX1,2,3 promoted the expression of dormant positively regulated genes such as sweet potato IbZEP, IbNCED3, IbDOG1, IbABI5, IbCKX3, and IbCKX7, which in turn delayed the sprouting of sweet potato rhizomes, and that PcCKX2 played a major role. We further screened three MYB transcription factors significantly associated with PcCKX1,2,3. Yeast one-hybrid, Dual-LUC, and EMSA experiments showed that PcMYB4, PcKUA1, and PcCSA all bind to and repress the expression of elements of the PcCKX1,2,3 promoter. Heterologous transformation of Arabidopsis experiments showed that PcMYB4, PcKUA1, and PcCSA repressed the expression of dormancy-associated genes such as DOG1, NCED3, ABI5, CKX3, and CKX7, which, in turn, facilitated Arabidopsis seed germination. Taken together, we found that PcMYBs are involved in the transcriptional regulation of PcCKXs to promote the germination of primary rhizome buds of P. cyrtonema. The results of this study lay the foundation for analyzing the molecular mechanism of primary rhizome bud germination in P. cyrtonema.
Keywords: Polygonatum cyrtonema Hua; 6-benzylaminopurine; Bud germination; Cytokinin oxidase; Primary rhizome; Transcriptional regulation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This manuscript does not involve any ethical or moral issues. All participants voluntarily participated in the study and were provided with detailed information about the study. Written informed consent was obtained from all participants prior to their enrollment. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Multiomics Reveals the Effect of Root Rot on Polygonati Rhizome and Identifies Pathogens and Biocontrol Strain.Microbiol Spectr. 2022 Apr 27;10(2):e0238521. doi: 10.1128/spectrum.02385-21. Epub 2022 Feb 28. Microbiol Spectr. 2022. PMID: 35225655 Free PMC article.
-
Transcriptomics and metabolomics changes triggered by exogenous 6-benzylaminopurine in relieving epicotyl dormancy of Polygonatum cyrtonema Hua seeds.Front Plant Sci. 2022 Jul 25;13:961899. doi: 10.3389/fpls.2022.961899. eCollection 2022. Front Plant Sci. 2022. PMID: 35958203 Free PMC article.
-
Genetic diversity and population structure of Polygonatum cyrtonema Hua in China using SSR markers.PLoS One. 2023 Aug 31;18(8):e0290605. doi: 10.1371/journal.pone.0290605. eCollection 2023. PLoS One. 2023. PMID: 37651363 Free PMC article.
-
Health benefits and application of polysaccharides from Polygonatum cyrtonema Hua.Front Pharmacol. 2025 Jul 18;16:1614596. doi: 10.3389/fphar.2025.1614596. eCollection 2025. Front Pharmacol. 2025. PMID: 40756982 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Hu Y, Tang Y, Zhang Z, et al. Recent advances in polysaccharides from the genus Polygonatum: Isolation, structures, bioactivities, and application. Food Hydrocolloids. 2023;140.
-
- Han C, Sun T, Liu Y, et al. Protective effect of Polygonatum sibiricum polysaccharides on gentamicin-induced acute kidney injury in rats via inhibiting p38 MAPK/ATF2 pathway. Int J Biol Macromol. 2020;151:595–601. - PubMed
MeSH terms
Substances
Grants and funding
- 03087060/the major project of Anhui Education Department
- YJS20210201/2021 Graduate Science Research Project in Anhui Province
- SKYJ202304/Anhui Science and Technology University Talent Introduction Project
- kj2021036/School Enterprise Scientific Research Project Collaboration
- 2022sysx033/Demonstration Experiment Training Center of Anhui Provincial Department of Education
LinkOut - more resources
Full Text Sources

