Modulation of retinal inflammation delays degeneration in a mouse model of geographic atrophy
- PMID: 40820160
- PMCID: PMC12358612
- DOI: 10.1038/s41598-025-15891-z
Modulation of retinal inflammation delays degeneration in a mouse model of geographic atrophy
Abstract
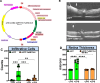
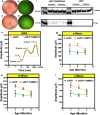
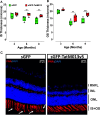
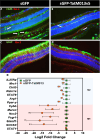
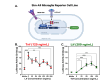
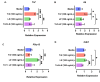
Geographic atrophy, the advanced form of age-related macular degeneration (AMD), is associated with increased oxidative stress and chronic inflammation. Pro-inflammatory genes, like TNF-α and IL-1β, are under the regulation of the transcription factor p65/RelA. We have previously shown that adeno-associated virus (AAV) delivery of the RelA inhibitory gene M013 blocks retinal inflammation in uveitis models. In this study, we evaluated the effects of RelA inhibition in an oxidative stress-driven geographic atrophy mouse model. We injected Sod2RPEcKO mice with rAAV, delivering either secreted GFP (sGFP control) or sGFP fused to a cell-penetrating version of the tagged M013 (sGFP-TatM013v5). Over nine months, we measured retinal function, structure, and morphological changes using electroretinography, optical coherence tomography, and fundoscopy. We quantified changes in inflammatory markers using multiplex ELISA, RT-qPCR, and immunofluorescence staining of the retinal tissue. Finally, we generated an NF-kB-luciferase reporter microglia cell line to study the impact of immune signaling changes on microglia. Mice injected with the rAAV delivering M013 had transient protection of their retinal function at 3 months. Based on ERG evaluations, the intravitreal injection of rAAV delivering sGFP-TatM013v5 significantly delayed the loss of retinal function. Furthermore, the rAAV-mediated expression of the sGFP-TatM013v5 protected photoreceptors' outer and inner segments based on OCT and immunofluorescence analysis. Analysis of postmortem tissues showed decreased migration of immune cells towards the RPE. Retinas injected with the sGFP-M013v5 vector showed increased levels of IL-9, IL10 and LIF. Finally, adding LIF to our NF-kB reporter cell line showed decreased TNF-induced reporter expression and modulation of microglia-specific genes. Our results indicate that modulating retinal inflammation could significantly slow the degeneration associated with geographic atrophy. Specifically, inhibiting the RelA protein in the retina may offer protective effects against retinal degeneration. Additionally, we demonstrated that LIF can counteract the influence of TNF on microglial gene expression. Future research will explore the dynamic interactions between RelA and other transcription factors and the NF-kB signaling pathway in the retina as they relate to retinal diseases.
Keywords: Age-related macular degeneration; Myxoma virus; NF-kB; Retinal inflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The University of Florida Institutional Animal Care and Use (IACUC) Board approved all the animal experiments conducted for this research (Protocol 201709854). All procedures adhered to ARVO guidelines for the use of animals in biomedical research. The study is reported in accordance with ARRIVE guidelines. Competing interests: The authors declare no competing interests.
Figures

Update of
-
Modulation of Retinal Inflammation Delays Degeneration in a Mouse Model of Geographic Atrophy.bioRxiv [Preprint]. 2023 Feb 9:2023.02.08.527757. doi: 10.1101/2023.02.08.527757. bioRxiv. 2023. Update in: Sci Rep. 2025 Aug 17;15(1):30153. doi: 10.1038/s41598-025-15891-z. PMID: 36798403 Free PMC article. Updated. Preprint.
Similar articles
-
Modulation of Retinal Inflammation Delays Degeneration in a Mouse Model of Geographic Atrophy.bioRxiv [Preprint]. 2023 Feb 9:2023.02.08.527757. doi: 10.1101/2023.02.08.527757. bioRxiv. 2023. Update in: Sci Rep. 2025 Aug 17;15(1):30153. doi: 10.1038/s41598-025-15891-z. PMID: 36798403 Free PMC article. Updated. Preprint.
-
Artificial intelligence for diagnosing exudative age-related macular degeneration.Cochrane Database Syst Rev. 2024 Oct 17;10(10):CD015522. doi: 10.1002/14651858.CD015522.pub2. Cochrane Database Syst Rev. 2024. PMID: 39417312
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Surgery for cataracts in people with age-related macular degeneration.Cochrane Database Syst Rev. 2017 Feb 16;2(2):CD006757. doi: 10.1002/14651858.CD006757.pub4. Cochrane Database Syst Rev. 2017. PMID: 28206671 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2015 Jan 7;1(1):CD008081. doi: 10.1002/14651858.CD008081.pub3. Cochrane Database Syst Rev. 2015. PMID: 25564068 Free PMC article.
References
-
- Jonas, J. B., Cheung, C. M. G. & Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia Pac. J. Ophthalmol.6, 493–497 (2017). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

