Feasibility of Deuterium Metabolic Magnetic Resonance Spectroscopy for the Investigation of Ischemia and Reperfusion in Rat Brain Slices Perfused Ex Vivo
- PMID: 40820229
- PMCID: PMC12358337
- DOI: 10.1002/nbm.70115
Feasibility of Deuterium Metabolic Magnetic Resonance Spectroscopy for the Investigation of Ischemia and Reperfusion in Rat Brain Slices Perfused Ex Vivo
Abstract
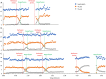
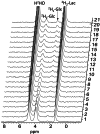
Investigating glucose metabolism in the brain using [6,6-2H2]glucose (2H2-Glc) and deuterium-based NMR spectroscopy has shown promise for noninvasive monitoring of the fate of this labeled compound. This approach has already been applied in vivo in small animals and human subjects. A model of perfused rat brain slices recently showed promise for the investigation of the metabolic consequences of acute ischemic stroke, which is a significant cause of death and morbidity worldwide. The current study aimed to implement the deuterium-based glucose metabolism monitoring approach to study the metabolic consequences of ischemia and reperfusion in the rat brain ex vivo. In agreement with previous studies, we found that deuterated lactate (2H2-Lac) was immediately formed in the brain upon administration of 2H2-Glc to the perfusion medium. This metabolite remained the predominant metabolic fate observed in the 2H-NMR spectra. Upon perfusion arrest, 2H2-Lac quickly built up to the same amount of 2H2-Glc eliminated from the medium engulfing the slices, reaching fivefold to sixfold its baseline level (n = 6, three animals, and two ischemic conditions in each). Upon reperfusion, 2H2-Lac decreased to its level before the ischemic condition, and 2H2-Glc returned to its baseline. 2H2-Lac washout to the medium amounted to 2.2% of the 2H2-Lac signal associated with the slices after about 5 h of perfusion with 2H2-Glc, suggesting that the 2H2-Lac signal observed during the experiments was predominantly intracellular. These results demonstrate the utility of 2H2-Glc and 2H-NMR in monitoring the consequences of ischemia and reperfusion in the perfused rat brain slices model.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Noninvasive 3-Dimensional 1 H-Magnetic Resonance Spectroscopic Imaging of Human Brain Glucose and Neurotransmitter Metabolism Using Deuterium Labeling at 3T : Feasibility and Interscanner Reproducibility.Invest Radiol. 2023 Jun 1;58(6):431-437. doi: 10.1097/RLI.0000000000000953. Epub 2023 Feb 4. Invest Radiol. 2023. PMID: 36735486 Free PMC article.
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
Assessing the degree of hepatic ischemia-reperfusion injury using physiologically based pharmacokinetic modeling of sodium fluorescein disposition in ex vivo machine-perfused livers.Am J Physiol Gastrointest Liver Physiol. 2024 Sep 1;327(3):G424-G437. doi: 10.1152/ajpgi.00048.2024. Epub 2024 Jun 25. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38917324 Free PMC article.
-
Cooling for cerebral protection during brain surgery.Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD006638. doi: 10.1002/14651858.CD006638.pub3. Cochrane Database Syst Rev. 2015. PMID: 25626888 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- WHO , “The Top 10 Causes of Death,” (2024), Retrieved: July 28, 2024, https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death.
-
- Siegel G. J., Agranoff B. W., and Albers R. W., Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed. ( Lippincott‐Raven , 1999).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

