An Aberrant Resurgence of Endogenous Retroviruses Prompts Myocarditis and Heart Failure
- PMID: 40820798
- PMCID: PMC12466171
- DOI: 10.1161/CIRCULATIONAHA.125.074845
An Aberrant Resurgence of Endogenous Retroviruses Prompts Myocarditis and Heart Failure
Abstract
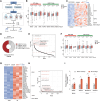
Background: Endogenous retroviruses (ERVs) occupy >8% of the human genome. Aberrant resurgence of ERVs has been implicated recently in several critical pathologies. However, the possible incidence and role of ERV resurgence in heart failure (HF), a leading cause of global morbidity and mortality, remain unexplored.
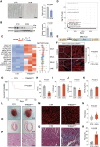
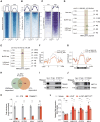
Methods: We established a total RNA sequencing analyzing pipeline to assess the ERV occurrence in human and murine HF models. We generated 2 myocardium-specific mouse lines by crossing Myh6-MerCreMer (Myosin heavy chain 6 promoter driving MerCreMer recombinase) with TRIM28f/f and SETDB1f/f mice to identify the molecular regulators of ERV resurgence and the downstream pathways in the heart. We evaluated ERV expression by total RNA sequencing, reverse transcription-quantitative polymerase chain reaction and RNA fluorescence in situ hybridization. We restrained ERV activation by overexpressing TRIM28 (tripartite motif-containing 28) using adeno-associated virus serotype 9. The therapeutic potential of the ERV-mediated inflammatory pathway was tested in a myocardial ischemia/reperfusion model.
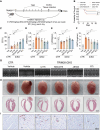
Results: ERVs, particularly class I ERVs, were prominently activated in multiple cross-species models of HF. Depletion of TRIM28, an epigenetic repressor, attenuated the epigenetic surveillance of trimethylation at lysine 9 of histone H3 and N6-methyladenosine, leading to the activation of ERVs in the failing heart. This ERV activation stimulated the antiviral innate immune pathways of TLR7/9 (Toll-like receptor 7/9) and NF-κB and lead to myocarditis and acute HF. Furthermore, restraining ERV activation and ERV-mediated innate immune responses by either adeno-associated virus serotype 9-mediated TRIM28 expression or a small-molecule TLR7/9 inhibitor improved heart function and alleviated HF in an ischemia/reperfusion model.
Conclusions: ERV resurgence is a specific molecular trait of HF, driven by TRIM28 depletion in cardiomyocytes. ERV resurgence activates the innate immune TLR7/9-NF-κB pathway and induces myocarditis and HF. Inception of ERVs and the ERV-mediated immune pathway confers cardiac protection. These results identify TRIM28-ERV-TLR7/9-NF-κB as a target for therapeutic management of myocarditis and HF.
Keywords: NF-κB; TRIM28; endogenous retrovirus; heart failure; myocarditis.
Conflict of interest statement
None.
Figures

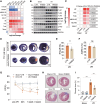
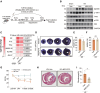
References
-
- Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272–3287. doi: 10.1093/cvr/cvac013 - PubMed
-
- Hernandez AF, Granger CB. Advancing care for acute heart failure--no time to relax. Lancet. 2009;373:1401–1402. doi: 10.1016/S0140-6736(09)60653-X - PubMed
-
- Lanciano S, Cristofari G. Measuring and interpreting transposable element expression. Nat Rev Genet. 2020;21:721–736. doi: 10.1038/s41576-020-0251-y - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

