Selective endo-Cyclic α-Functionalization of Saturated N-Alkyl Piperidines
- PMID: 40820907
- PMCID: PMC12400421
- DOI: 10.1021/acs.joc.5c01742
Selective endo-Cyclic α-Functionalization of Saturated N-Alkyl Piperidines
Abstract
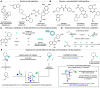
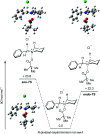
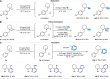
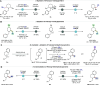
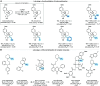
Saturated N-alkyl heterocycles are among the most significant structural motifs in natural products, small-molecule biological probes, and pharmaceutical agents, as evidenced by their prevalence in FDA-approved drugs. Substituted derivatives of these cyclic tertiary alkylamine scaffolds often exhibit markedly different physicochemical and biological properties compared to their unsubstituted counterparts. Consequently, methods for the selective functionalization of these scaffolds would greatly facilitate the optimization of biological activity, physicochemical properties, and systematic evaluations of structure-activity relationships. In this work, we present a robust platform for the late-stage α-functionalization of N-alkyl piperidines through a sequential process involving iminium ion formation followed by nucleophilic functionalization. Key to this strategy is the selective formation of endo-iminium ions from six-membered N-heterocycles, achieved via α-C-H elimination of cyclic tertiary alkylamine N-oxides. This approach provides exceptional endo-selectivity, enabling efficient further functionalization. The method allows for the in situ addition of diverse carbon-based nucleophiles to the iminium intermediates, demonstrated across a range of piperidine-based systems; alkylation, azinylation, and trifluoromethylation are successfully demonstrated through a variety of activation modes. Furthermore, the formal C-H functionalization sequence has been successfully applied to the late-stage modification of complex bioactive molecules, underscoring the potential of this methodology to expand drug-like chemical space.
Figures




References
-
- Coleman P. J., Schreier J. D., Cox C. D., Breslin M. J., Whitman D. B., Bogusky M. J., McGaughey G. B., Bednar R. A., Lemaire W., Doran S. M.. et al. Discovery of [(2R,5R)-5-{[(5-Fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2-(pyrimidin-2-yl)phenyl]methanone (MK-6096): A Dual Orexin Receptor Antagonist with Potent Sleep-Promoting Properties. ChemMedChem. 2012;7:415–424. doi: 10.1002/cmdc.201200025. - DOI - PubMed
- Schönherr H., Cernak T.. Profound Methyl Effects in Drug Discovery and a Call for New C–H Methylation Reactions. Angew. Chem., Int. Ed. 2013;52:12256–12267. doi: 10.1002/anie.201303207. - DOI - PubMed
- Hu X.-G., Hunter L.. Stereoselectively fluorinated N-heterocycles: a brief survey. Beilstein J. Org. Chem. 2013;9:2696–2708. doi: 10.3762/bjoc.9.306. - DOI - PMC - PubMed
- St. Jean D. J. Jr., Fotsch C.. Mitigating Heterocycle Metabolism in Drug Discovery. J. Med. Chem. 2012;55:6002–6020. doi: 10.1021/jm300343m. - DOI - PubMed
- Morgenthaler M., Schweizer E., Hoffmann-Röder A., Benini F., Martin R. E., Jaeschke G., Wagner B., Fischer H., Bendels S., Zimmerli D.. et al. Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem. 2007;2:1100–1115. doi: 10.1002/cmdc.200700059. - DOI - PubMed
- Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A.. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. - DOI - PubMed
- Mainolfi N., Ehara T., Karki R. G., Anderson K., Mac Sweeney A., Liao S.-M., Argikar U. A., Jendza K., Zhang C., Powers J.. et al. Discovery of 4-((2S,4S)-4-Ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl)benzoic Acid (LNP023), a Factor B Inhibitor Specifically Designed To Be Applicable to Treating a Diverse Array of Complement Mediated Diseases. J. Med. Chem. 2020;63:5697–5722. doi: 10.1021/acs.jmedchem.9b01870. - DOI - PubMed
-
- Dutta S., Li B., Rickertsen D. R. L., Valles D. A., Seidel D.. C-H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods. SynOpen. 2021;5:173–228. doi: 10.1055/s-0040-1706051. - DOI - PMC - PubMed
- Chen W., Seidel D.. Condensation-Based Methods for the C-H Bond Functionalization of Amines. Synthesis. 2021;53:3869–3908. doi: 10.1055/a-1631-2140. - DOI - PMC - PubMed
- Antermite D., Bull J.. Transition Metal-Catalyzed Directed C(sp3)–H Functionalization of Saturated Heterocycles. Synthesis. 2019;51:3171–3204. doi: 10.1055/s-0037-1611822. - DOI
-
- Beak P., Lee W.-K.. α-Lithioamine synthetic equivalents from dipole-stabilized carbanions: The t-Boc group as an activator for α′-lithiation of carbamates. Tetrahedron Lett. 1989;30:1197–1200. doi: 10.1016/S0040-4039(00)72714-6. - DOI
- Klapars A., Campos K. R., Waldman J. H., Zewge D., Dormer P. G., Chen C.-y.. Enantioselective Pd-Catalyzed α-Arylation of N-Boc-Pyrrolidine: The Key to an Efficient and Practical Synthesis of a Glucokinase Activator. J. Org. Chem. 2008;73:4986–4993. doi: 10.1021/jo8006804. - DOI - PubMed
- Chatani N., Asaumi T., Ikeda T., Yorimitsu S., Ishii Y., Kakiuchi F., Murai S.. Carbonylation at sp3 C–H Bonds Adjacent to a Nitrogen Atom in Alkylamines Catalyzed by Rhodium Complexes. J. Am. Chem. Soc. 2000;122:12882–12883. doi: 10.1021/ja002561w. - DOI
- Verma P., Richter J. M., Chekshin N., Qiao J. X., Yu J.-Q.. Iridium(I)-Catalyzed α-C(sp3)–H Alkylation of Saturated Azacycles. J. Am. Chem. Soc. 2020;142(11):5117–5125. doi: 10.1021/jacs.9b12320. - DOI - PMC - PubMed
- Davies H. M. L., Venkataramani C., Hansen T., Hopper D. W.. New Strategic Reactions for Organic Synthesis: Catalytic Asymmetric C–H Activation α to Nitrogen as a Surrogate for the Mannich Reaction. J. Am. Chem. Soc. 2003;125:6462–6468. doi: 10.1021/ja0290072. - DOI - PubMed
- Suga S., Okajima M., Yoshida J.-i.. Reaction of an electrogenerated ‘iminium cation pool’ with organometallic reagents. Direct oxidative α-alkylation and -arylation of amine derivatives. Tetrahedron Lett. 2001;42:2173–2176. doi: 10.1016/S0040-4039(01)00128-9. - DOI
- Novaes L. F. T., Ho J. S. K., Mao K., Liu K., Tanwar M., Neurock M., Villemure E., Terrett J. A., Lin S.. Exploring Electrochemical C(sp3)–H Oxidation for the Late-Stage Methylation of Complex Molecules. J. Am. Chem. Soc. 2022;144:1187–1197. doi: 10.1021/jacs.1c09412. - DOI - PubMed
- Chen W., Ma L., Paul A., Seidel D.. Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem. 2018;10:165–169. doi: 10.1038/nchem.2871. - DOI - PMC - PubMed
- Zuo Z., MacMillan D. W. C.. Decarboxylative Arylation of α-Amino Acids via Photoredox Catalysis: A One-Step Conversion of Biomass to Drug Pharmacophore. J. Am. Chem. Soc. 2014;136:5257–5260. doi: 10.1021/ja501621q. - DOI - PMC - PubMed
- Johnston C. P., Smith R. T., Allmendinger S., MacMillan D. W. C.. Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides. Nature. 2016;536:322–325. doi: 10.1038/nature19056. - DOI - PMC - PubMed
- Shaaban S., Maulide N.. Metal-Free Redox Transformations for C–C and C–N Bond Construction. Synlett. 2017;28:2707–2713. doi: 10.1055/s-0036-1588776. - DOI
- Shaw M. H., Shurtleff V. W., Terrett J. A., Cuthbertson J. D., MacMillan D. W. C.. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. - DOI - PMC - PubMed
- McManus J. B., Onuska N. P. R., Nicewicz D. A.. Generation and Alkylation of α-Carbamyl Radicals via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2018;140:9056–9060. doi: 10.1021/jacs.8b04890. - DOI - PubMed
- Bhatt K., Adili A., Tran A. H., Elmallah K. M., Ghiviriga I., Seidel D.. Photocatalytic Decarboxylative Alkylation of Cyclic Imine–BF3 Complexes: A Modular Route to Functionalized Azacycles. J. Am. Chem. Soc. 2024;146:26331–26339. doi: 10.1021/jacs.4c08754. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

