Contrasting Behavioural and Biochemical Characteristics of Normal and Spontaneously α-Synuclein-Deficient Mice Treated With MPTP
- PMID: 40820918
- PMCID: PMC12359103
- DOI: 10.1111/jnc.70201
Contrasting Behavioural and Biochemical Characteristics of Normal and Spontaneously α-Synuclein-Deficient Mice Treated With MPTP
Abstract
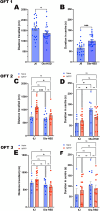
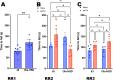
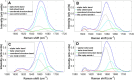
α-Synuclein is the primary toxic constituent of Lewy bodies, but its exact function under homeostatic conditions remains elusive. To better understand the role of α-synuclein, we compared two C57BL sub-strains: the normal α-synuclein-expressing J6 and the α-synuclein-deficient J6-OlaHSD, for behavioural, dopaminergic and glial integrity in substantia nigra (SN) and caudate putamen (CPu) before and after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. After MPTP treatment, J6 mice showed significant weight loss (-7% by Day 10), whereas OlaHSD mice maintained stable body weight. At baseline, J6 mice exhibited 33% higher locomotor activity but 38% more thigmotaxis, and 33% less endurance on the Rotarod test than OlaHSD mice. Loss of tyrosine hydroxylase-positive neurons was similar in OlaHSD (-40%) and J6 mice (-34%). J6 mice had double the SN GFAP-ir cells of J6-OlaHSD, a difference that was unchanged by MPTP treatment. In the CPu, MPTP increased GFAP-ir cells in both strains, but Iba1-ir cells significantly increased only in MPTP-treated OlaHSD mice, compared to J6 strain. We further compared the biochemical signatures using Raman micro-spectroscopy. The Raman spectra of the freshly cut SN sections showed a greater shift in the α-helix to β-sheet protein conformation ratio in MPTP-induced J6 mice, likely due to the absence of the Snca1 gene in OlaHSD mice. These findings suggest that the absence of α-synuclein plays a subtle role in the behavioural and neurochemical differences but has no significant effect on dopaminergic neurotransmission. It is therefore concluded that the presence of α-synuclein is important for non-dopaminergic behaviours such as anxiety-like behaviours and regulation of body weight. Under toxic challenge, gliosis in the SN and CPu may be regulated by α-synuclein. This study also emphasises the utility of Raman spectroscopy as a potential tool for identifying subtle protein conformation differences in mice with and without Snca1.
Keywords: 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP); GFAP; IBA‐1; Parkinson's disease model; Raman spectroscopy; astrocytes; dopamine neuron; microglia; α‐synuclein; α‐synuclein deficiency.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Double Toxic MPTP+CBE Presymptomatic Parkinson-Like Phenotype in Mice.Biochemistry (Mosc). 2025 Aug;90(8):1049-1063. doi: 10.1134/S000629792560070X. Biochemistry (Mosc). 2025. PMID: 40886386
-
Foliglurax, a positive allosteric modulator of the metabotrophic glutamate receptor 4, protects dopaminergic neurons in MPTP-lesioned male mice.Brain Res. 2023 Jun 15;1809:148349. doi: 10.1016/j.brainres.2023.148349. Epub 2023 Mar 25. Brain Res. 2023. PMID: 36972837
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Agirman, G. , Yu K. B., and Hsiao E. Y.. 2021. “Signaling Inflammation Across the Gut‐Brain Axis.” Science 374, no. 6571: 1087–1092. - PubMed
-
- Apetri, M. M. , Maiti N. C., Zagorski M. G., Carey P. R., and Anderson V. E.. 2006. “Secondary Structure of α‐Synuclein Oligomers: Characterization by Raman and Atomic Force Microscopy.” Biochemical Journal 355, no. 1: 63–71. - PubMed
-
- Ayton, S. , George J. L., Adlard P. A., Bush A. I., Cherny R. A., and Finkelstein D. I.. 2013. “The Effect of Dopamine on MPTP‐Induced Rotarod Disability.” Neuroscience Letters 543: 105–109. - PubMed
-
- Chandra, S. , Gallardo G., Fernández‐Chacón R., Schlüter O. M., and Südhof T. C.. 2005. “α‐Synuclein Cooperates With CSPα in Preventing Neurodegeneration.” Cell 123, no. 3: 383–396. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

