Crosstalk between the circadian clock, intestinal stem cell niche, and epithelial cell fate decision
- PMID: 40821111
- PMCID: PMC12354785
- DOI: 10.1016/j.gendis.2025.101650
Crosstalk between the circadian clock, intestinal stem cell niche, and epithelial cell fate decision
Abstract
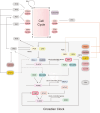
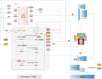
The circadian rhythm, a 24-h cycle, plays a crucial role in regulating gut physiological processes, particularly the proliferation and differentiation of intestinal epithelial cells, which are essential for gut homeostasis and repair. This review discusses the complex interactions between circadian rhythms, cell cycle regulation, and key signaling pathways (Wnt, Notch, and Hippo) in the context of the intestinal stem cell niche and epithelial cell fate decisions. Key molecules such as brain and muscle ARNT-like 1 (BMAL1), circadian locomotor output cycles kaput (CLOCK), hairy and enhancer of split 1 (Hes1), and Yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) coordinate stem cell functions with circadian rhythms. We discuss how Notch signaling regulates the cell cycle and interacts with circadian rhythms. Additionally, we explore the role of Hippo-Wnt signaling in balancing cell proliferation and differentiation. Furthermore, we highlight the intricate relationships between circadian clock components and signaling pathways, emphasizing the importance of temporal coordination in determining epithelial cell fate. We also discuss shared enzymes, including casein kinase 1 delta (CK1δ), glycogen synthase kinase 3 (GSK3), and AMP-activated protein kinase (AMPK), which play a role in regulating the cell cycle, circadian rhythm, and signaling pathways. In summary, this review offers valuable insights into the regulatory mechanisms that control stem cell behavior and epithelial cell differentiation, suggesting promising directions for future research in intestinal biology and tissue homeostasis.
Keywords: Cell cycle regulation; Circadian clock; Epithelial differentiation; Intestinal stem cell; Signaling pathways.
© 2025 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
All authors reported no conflict of interests in this work.
Figures



Similar articles
-
The molecular circadian clock of eosinophils: a potential therapeutic target for asthma.Am J Physiol Cell Physiol. 2025 May 1;328(5):C1394-C1408. doi: 10.1152/ajpcell.00149.2025. Epub 2025 Mar 25. Am J Physiol Cell Physiol. 2025. PMID: 40131242 Free PMC article.
-
The Pro-Apoptotic Effect of Glucose Restriction in NSCLC via AMPK-Regulated Circadian Clock Gene Bmal1.Cancer Sci. 2025 Aug;116(8):2101-2112. doi: 10.1111/cas.70098. Epub 2025 May 20. Cancer Sci. 2025. PMID: 40394734 Free PMC article.
-
A common alteration in effort-based decision-making in apathy, anhedonia, and late circadian rhythm.Elife. 2025 Jun 16;13:RP96803. doi: 10.7554/eLife.96803. Elife. 2025. PMID: 40522113 Free PMC article.
-
The cross-talk between leptin and circadian rhythm signaling proteins in physiological processes: a systematic review.Mol Biol Rep. 2023 Dec;50(12):10427-10443. doi: 10.1007/s11033-023-08887-3. Epub 2023 Oct 24. Mol Biol Rep. 2023. PMID: 37874505
-
Neurobiology of the circadian clock and its role in cardiovascular disease: Mechanisms, biomarkers, and chronotherapy.Neurobiol Sleep Circadian Rhythms. 2025 Jun 3;19:100131. doi: 10.1016/j.nbscr.2025.100131. eCollection 2025 Nov. Neurobiol Sleep Circadian Rhythms. 2025. PMID: 40534620 Free PMC article. Review.
References
-
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. - PubMed
-
- Ayyaz A., Kumar S., Sangiorgi B., et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019;569(7754):121–125. - PubMed
-
- Qi Z., Chen Y.G. Regulation of intestinal stem cell fate specification. Sci China Life Sci. 2015;58(6):570–578. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

