Reirradiation of recurrent glioblastoma: Results from a single-center retrospective cohort study
- PMID: 40821395
- PMCID: PMC12357250
- DOI: 10.1016/j.ctro.2025.101029
Reirradiation of recurrent glioblastoma: Results from a single-center retrospective cohort study
Abstract
Purpose: The management of recurrent glioblastoma (rGBM) remains a clinical challenge, with only limited therapeutic options available to date. Reirradiation may offer a progression-free survival (PFS) benefit in selected cases, but data are scarce.
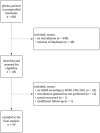
Methods: Consecutive patients from the last 10 years with GBM (CNS WHO grade 4, IDH-wildtype) who underwent at least one additional course of cranial radiotherapy for suspected or histopathologically confirmed rGBM at a tertiary neuro-oncological center were retrospectively analyzed. The primary endpoint was PFS, secondary endpoints included reirradiation-related adverse event rates, with a particular focus on radiation necrosis (RN).
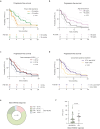
Results: Fifty-nine patients were included with a median follow-up (range) of 8.7 (0.5-48.0) months after reirradiation. The median time to first recurrence was 15 (4-89) months, with the majority occurring in-field (59.7 %). The EQD2⍺/β=10 ranged from 31.3-80.2 Gy with a median prescription dose of 42 Gy. Reirradiation was combined with systemic therapy in 81.4 % of patients. No grade 3-5 acute reirradiation-related adverse events were observed. RN was diagnosed in 16.9 % of patients (80 % grade 2 and 20 % grade 3), with a notably low rate in those receiving anti-VEGF therapy parallel to reirradiation. RN risk was independent of reirradiation volume or dose (p = 0.15 and 0.43, respectively). The disease control rate following reirradiation was 83.6 % and the median PFS was 5.9 (0.5-48.0) months. Concomitant chemotherapy or anti-VEGF therapy was significantly associated with improved outcomes (p = 0.049), whereas smaller reirradiation volumes demonstrated a non-significant trend towards longer PFS (p = 0.23).
Conclusion: In this retrospective analysis, reirradiation for rGBM was feasible and safe, conferring a potential PFS benefit in selected patients. Bevacizumab emerged as a particularly promising combination partner, contributing to both RN prevention and enhanced efficacy.
Keywords: Glioblastoma; Radiation necrosis; Recurrence; Reirradiation.
© 2025 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: FAG reports travel expenses, stocks, and honoraria from TME Pharma AG; research grants and travel expenses from ELEKTA AB; grants, research grants, travel expenses, and honoraria from Carl Zeiss Meditec AG; grants, research grants, travel expenses, and honoraria from OncoMANGETx, Inc.; travel expenses and research grants from Varian Medical Systems, Inc.; travel expenses and/or honoraria from Bristol-Myers Squibb, Cureteq AG, Roche Pharma AG, MSD Sharp and Dohme GmbH, Siemens Healthineers AG, Varian Medical Systems, Inc., and AstraZeneca GmbH; non-financial support from Oncare GmbH and Opasca GmbH; patent US10857388B2 together with Carl Zeiss Meditec AG, and patent EP4119191A1 with the University of Heidelberg. The remaining authors report no conflict of interest related to this work
Figures
References
-
- Stupp R., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Andratschke N., et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus on re-irradiation: definition, reporting, and clinical decision making. Lancet Oncol. 2022;23:e469–e478. - PubMed
LinkOut - more resources
Full Text Sources

