Use of Intrapleural Alteplase in the Treatment of Parapneumonic Effusion in Children: A Report of a 10-year Experience
- PMID: 40821423
- PMCID: PMC12351476
- DOI: 10.5863/JPPT-24-00057
Use of Intrapleural Alteplase in the Treatment of Parapneumonic Effusion in Children: A Report of a 10-year Experience
Abstract
Objectives: Intrapleural alteplase is used in children with parapneumonic effusion (PPE) with variable dosing strategies. We compared the outcomes of a lower (≤2 mg) and a higher (>2 mg) alteplase dose in children with PPE.
Methods: A retrospective study was conducted among admitted patients younger than 18 years who received at least 1 intrapleural alteplase dose from July 2014 to May 2023. The primary outcome was the treatment failure rate. Secondary outcomes included chest tube output and duration of placement and hospital and pediatric intensive care unit (PICU) length of stays.
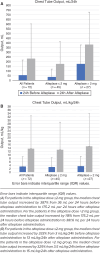
Results: Seventy-two patients were included (lower dose: 62.5% vs higher dose: 37.5%) with a median age of 5 years (IQR, 1-8 years). The median alteplase dose was 2 mg (IQR, 2-4 mg). Treatment failure occurred in 10 (14%) patients. The lower dose group had a similar failure rate compared with the higher dose group (lower dose: 9% vs higher dose: 22%; p = 0.161), despite a statistically significant higher median chest tube output in the higher dose group (346 [IQR, 256-466] vs 175 [IQR, 70-358] mL/24h; p = 0.002). However, after adjusting for weight, both groups had a similar output (12 mL/kg/24h). Alteplase instillation after primary video-assisted thoracoscopic surgery (VATS) was associated with a significant reduction in the duration of chest tube placement and hospital and PICU stays.
Conclusions: Lower alteplase doses (≤2 mg) were effective for most children with PPE. Alteplase combined with primary VATS might be associated with better outcomes.
Keywords: VATS; alteplase; chest tube; children; empyema; parapneumonic effusion.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org.
Figures
Similar articles
-
Surgical versus non-surgical management for pleural empyema.Cochrane Database Syst Rev. 2017 Mar 17;3(3):CD010651. doi: 10.1002/14651858.CD010651.pub2. Cochrane Database Syst Rev. 2017. PMID: 28304084 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Caffeine dosing regimens in preterm infants with or at risk for apnea of prematurity.Cochrane Database Syst Rev. 2023 Apr 11;4(4):CD013873. doi: 10.1002/14651858.CD013873.pub2. Cochrane Database Syst Rev. 2023. PMID: 37040532 Free PMC article. Review.
References
-
- de Benedictis FM, Kerem E, Chang AB et al. Complicated pneumonia in children. Lancet. 2020;396(10253):786–798. - PubMed
-
- Hendaus MA, Janahi IA. Parapneumonic effusion in children: an up-to-date review. Clin Pediatr (Phila) 2016;55(1):10–18. - PubMed
-
- Yu D, Buchvald F, Brandt B, Nielsen KG. Seventeen-year study shows rise in parapneumonic effusion and empyema with higher treatment failure after chest tube drainage. Acta Paediatr. 2014;103(1):93–99. - PubMed
LinkOut - more resources
Full Text Sources