Synthesis and Evaluation of 2‑Substituted Quinazolin-4(3 H)‑ones as Potential Antileukemic Agents
- PMID: 40821533
- PMCID: PMC12355269
- DOI: 10.1021/acsomega.5c04106
Synthesis and Evaluation of 2‑Substituted Quinazolin-4(3 H)‑ones as Potential Antileukemic Agents
Abstract
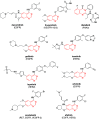
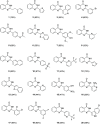
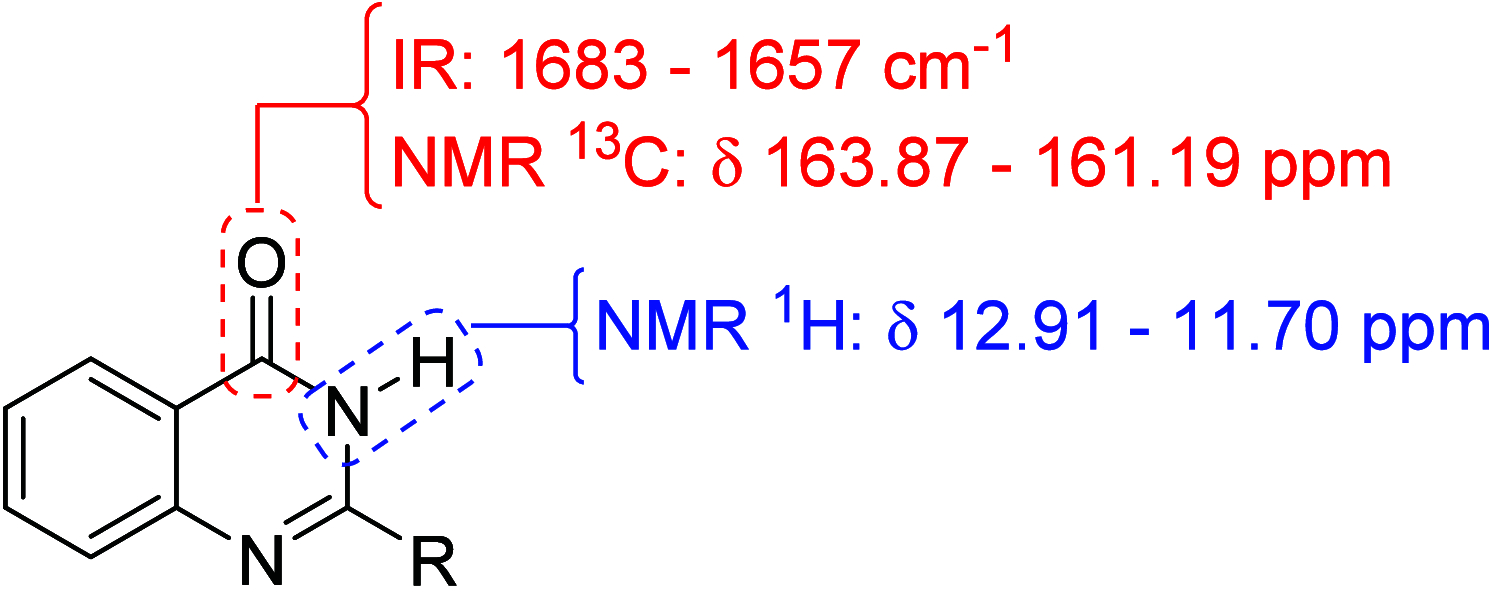
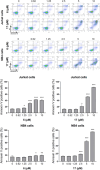
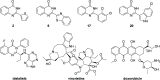
The increasing life expectancy and rising prevalence of cancer emphasize the need for innovative therapeutic strategies. Targeted therapies have revolutionized cancer treatment by offering greater specificity and reduced toxicity compared to traditional cytotoxic drugs. Acute leukemias, including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), remain aggressive malignancies with poor outcomes, particularly in elderly patients. Despite advancements, resistance to chemotherapy and adverse effects necessitate the discovery of novel antitumor compounds. Quinazolines, a versatile class of heterocyclic compounds, exhibit diverse biological activities, including anticancer properties. In this study, 20 derivatives of 2-substituted quinazolin-4-(3H)-ones were synthesized via condensation of 2-aminobenzamide with aldehydes in dimethyl sulfoxide. The compounds were characterized using IR, 1H NMR, and 13C NMR spectroscopy. Biological evaluation revealed that compounds 6 and 17 exhibited potent cytotoxic effects against T cell ALL (jurkat cells) and AML of promyelocytic subtype (APL) NB4 cells, with compound 17 showing IC50 values below 5 μM in both cell types. Compound 6 demonstrated selectivity for Jurkat cells. Further in vitro analyses, including apoptosis/cycle cell assays and pharmacokinetic predictions, confirmed their therapeutic potential. The data open new perspectives for "in vivo" studies concerning the application of quinazolin-4-(3H)-ones in treatment of acute leukemias of lymphoid and myeloid origins.
© 2025 The Authors. Published by American Chemical Society.
Figures
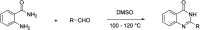


References
-
- Huang G., Guo F., Liu L., Taksa L., Cheng Z., Tani M., Zimmermann K. F., Franklin M., Silva S. S. M.. Changing Impact of COVID-19 on Life Expectancy 2019–2023 and Its Decomposition: Findings from 27 Countries. SSM - Popul. Health. 2024;25:101568. doi: 10.1016/j.ssmph.2023.101568. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
