Subcritical Water-Driven Complete Mineralization of Silane Coupling Agents with Short Perfluoroalkyl Chains
- PMID: 40821544
- PMCID: PMC12355435
- DOI: 10.1021/acsomega.5c04738
Subcritical Water-Driven Complete Mineralization of Silane Coupling Agents with Short Perfluoroalkyl Chains
Abstract
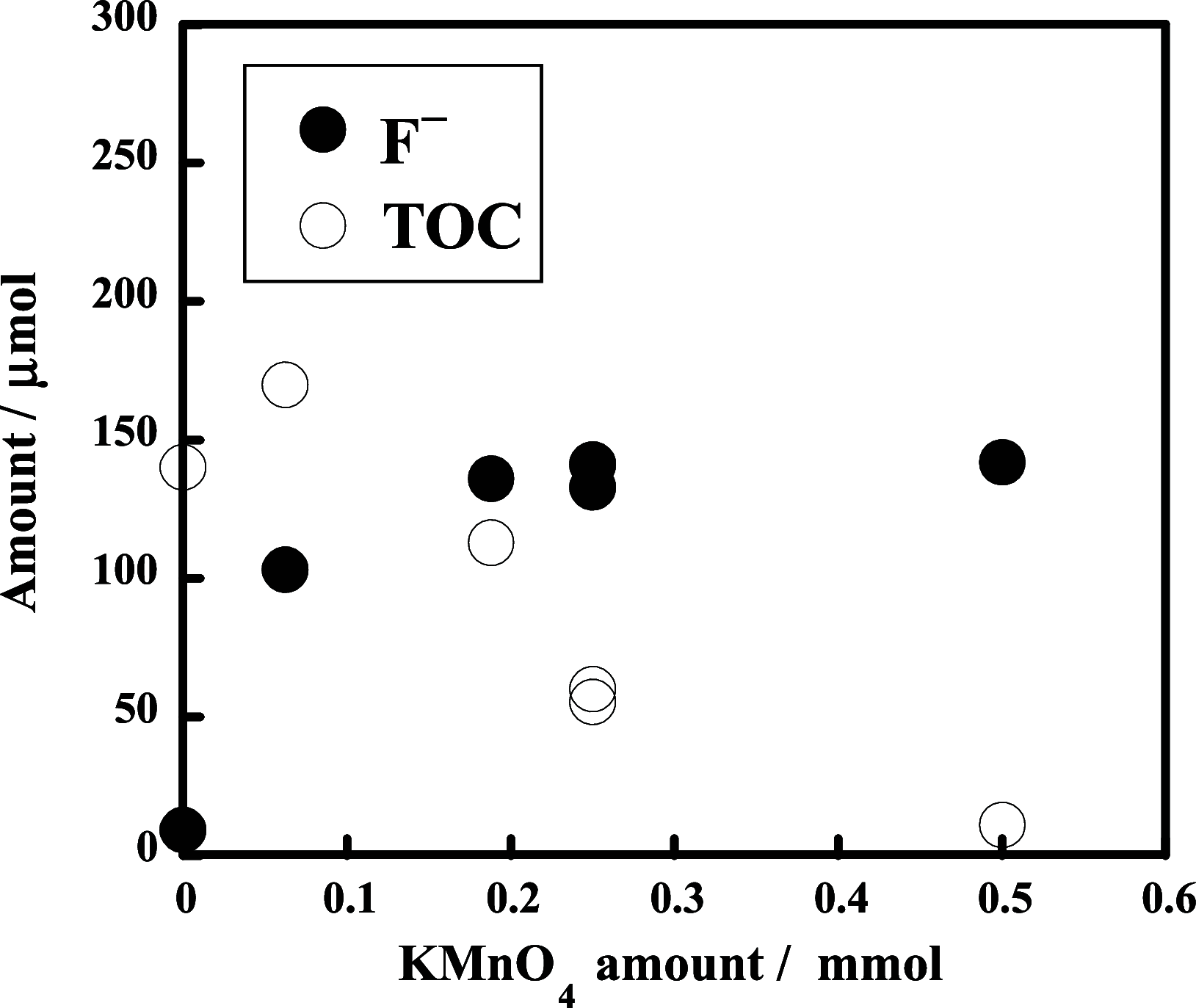
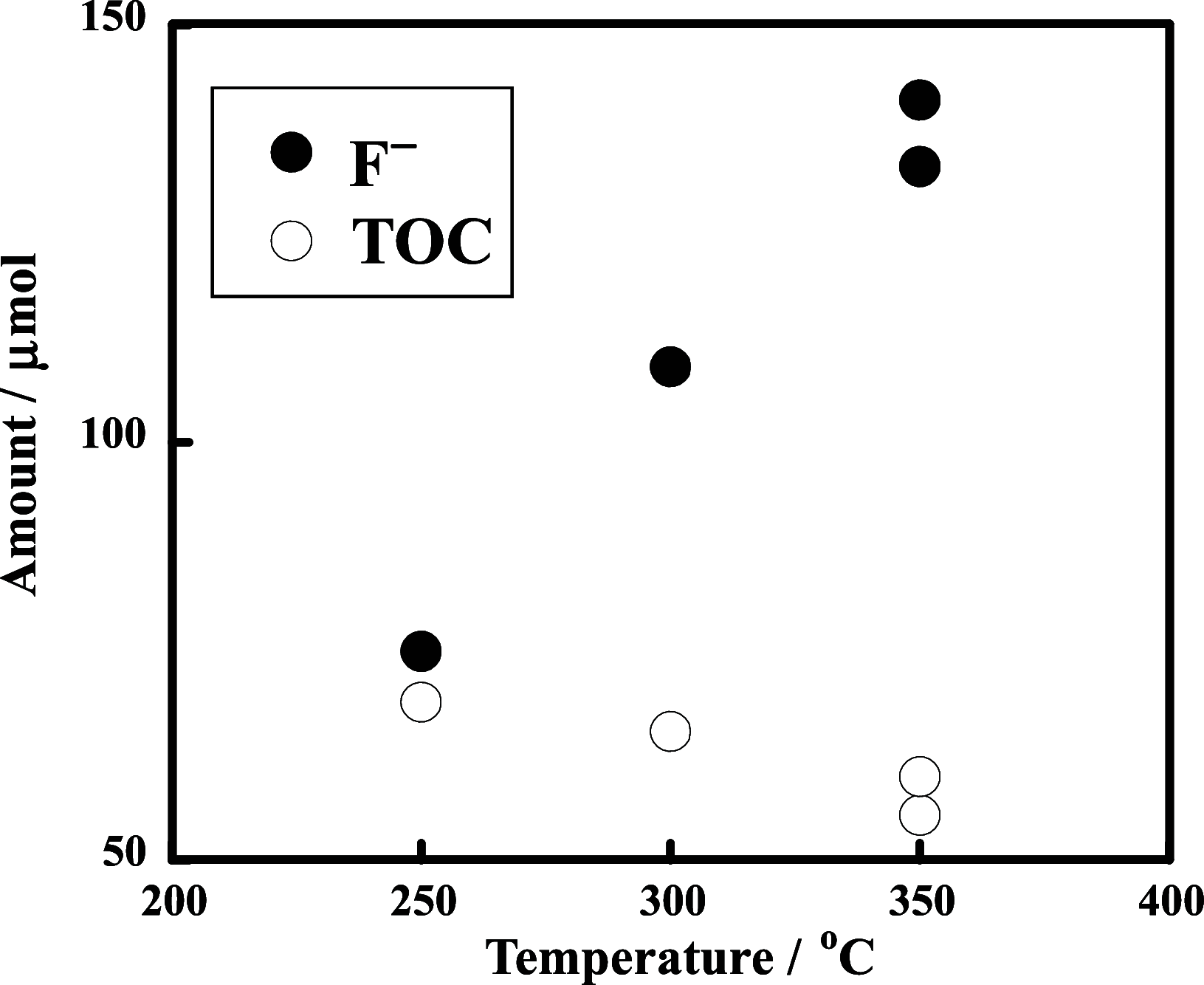
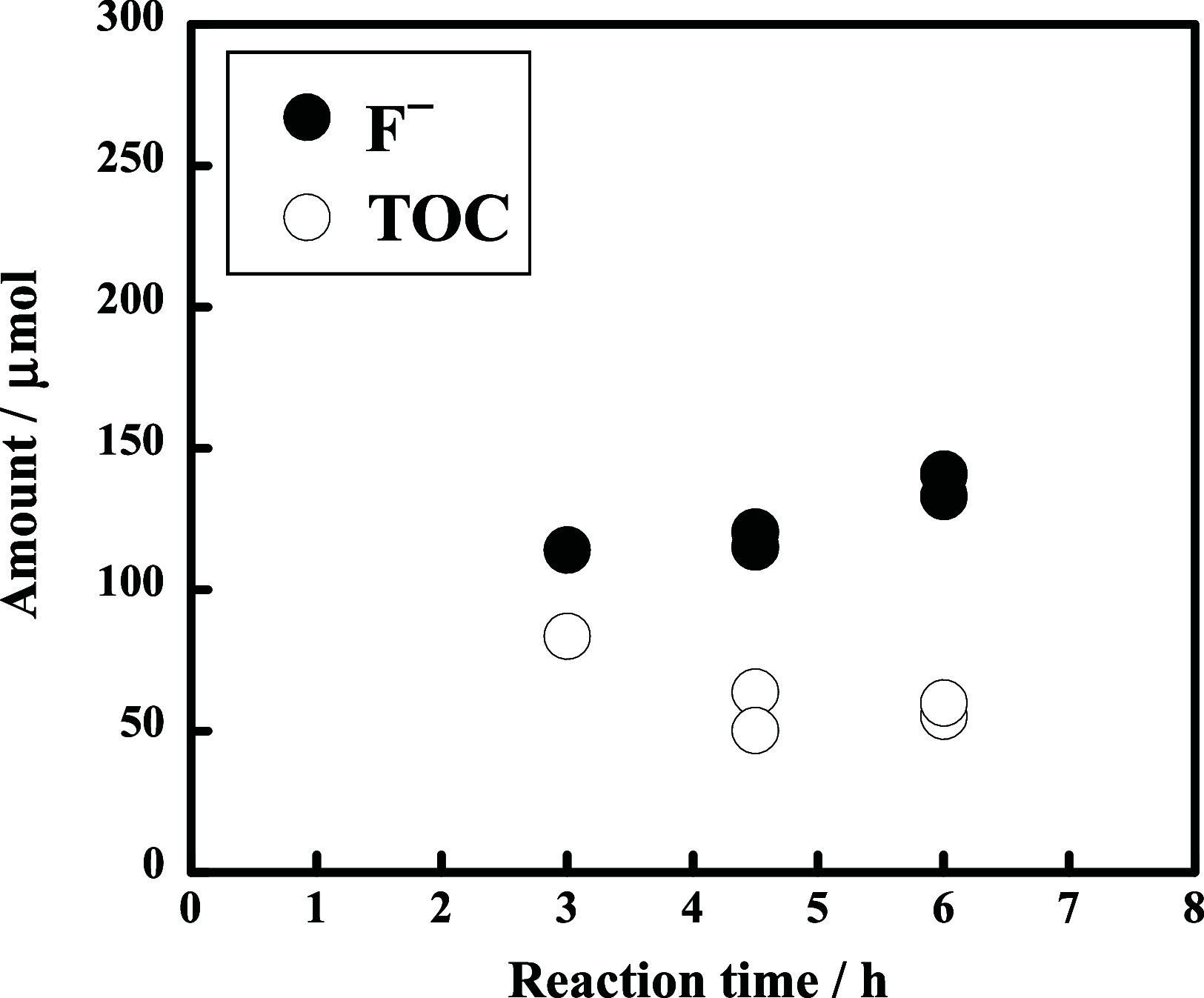
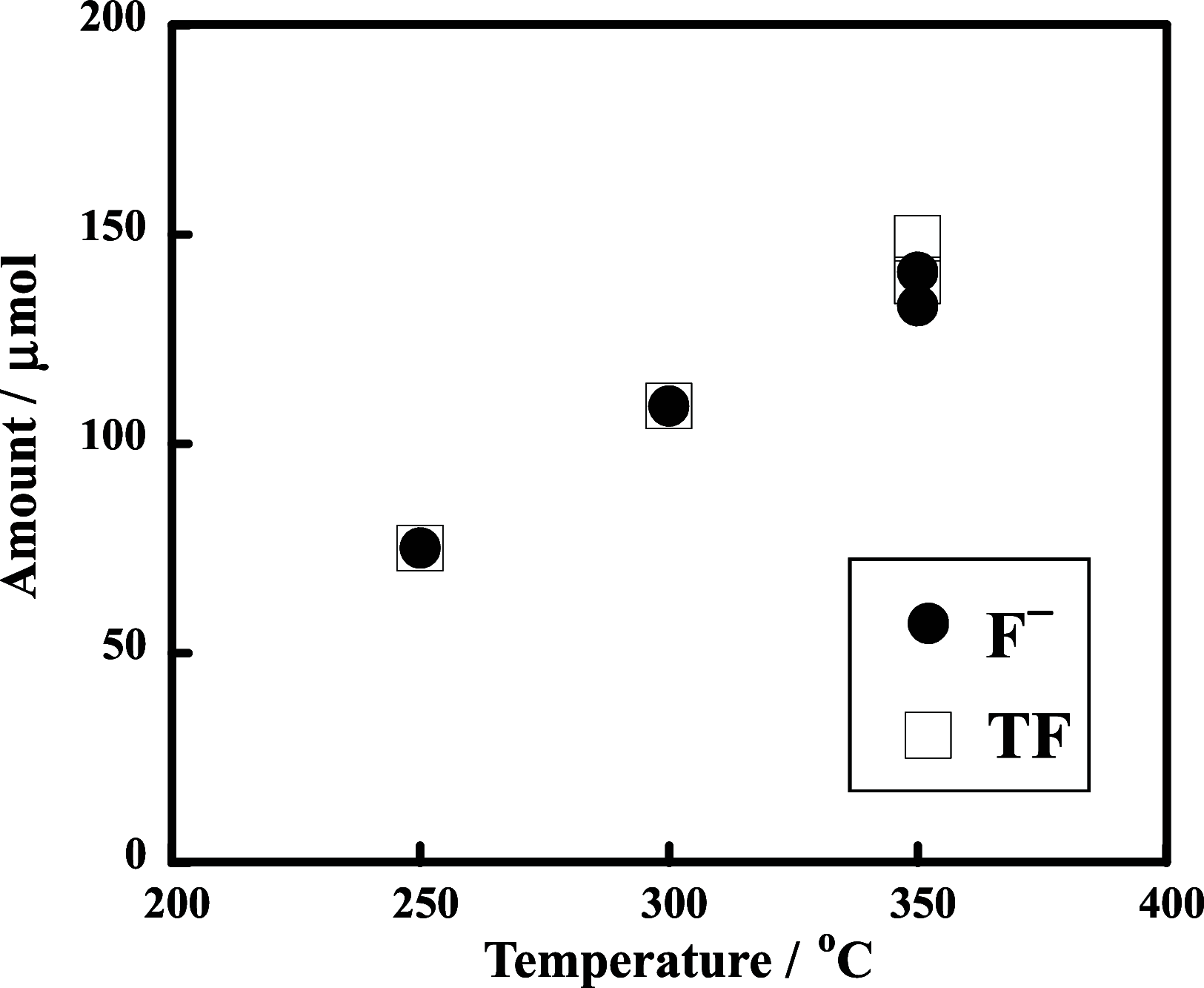
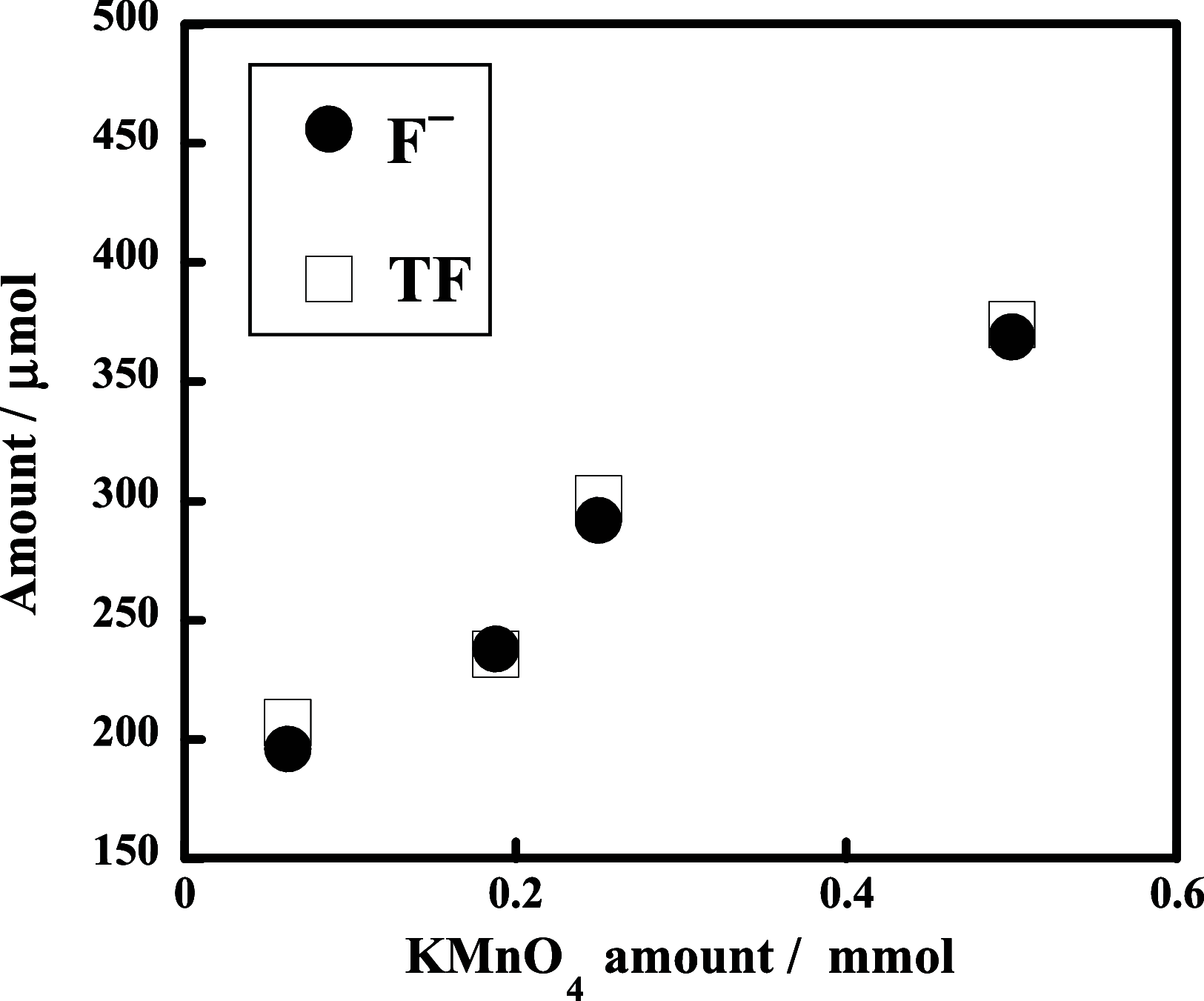
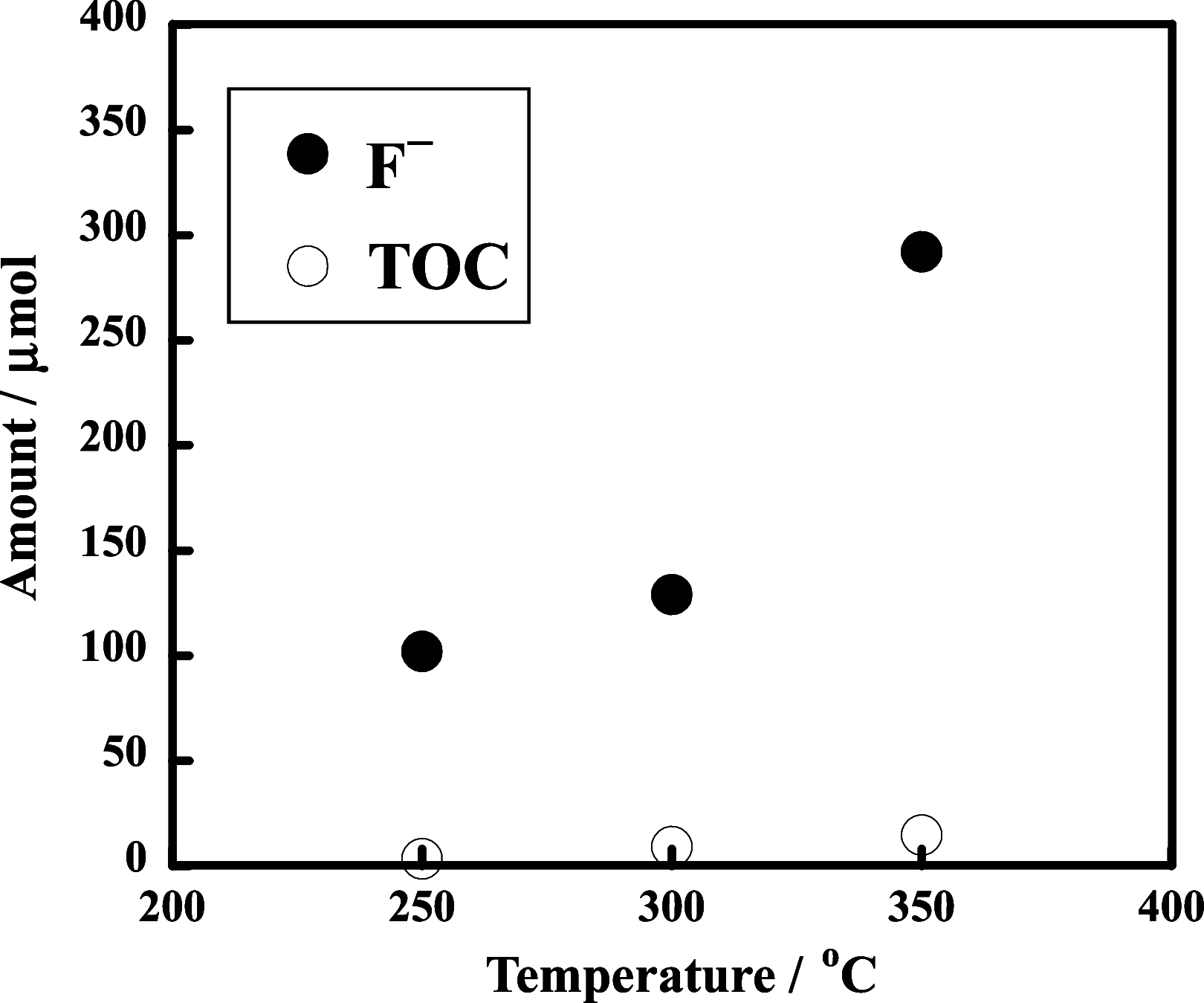
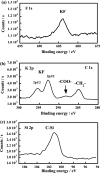
The decomposition of CF3CH2CH2Si-(OMe)3 and C4F9CH2CH2Si-(OMe)3typical fluorinated silane coupling agents used for surface modificationwas investigated in subcritical water for potential waste treatment applications. When CF3CH2CH2Si-(OMe)3 was heated in subcritical water with no additive at 350 °C, only a small amount of fluoride (F-) was produced. In contrast, virtually complete mineralization of CF3CH2CH2Si-(OMe)3 was achieved in the presence of KMnO4. Specifically, after treatment with 0.50 mmol of KMnO4 for 6 h, the F- yield reached 100%, and the residual total organic carbon in the reaction solution was reduced to 4% of the initial carbon content of CF3CH2CH2Si-(OMe)3. Furthermore, under the same reaction conditions, quasi-complete mineralization of C4F9CH2CH2Si-(OMe)3 was also attained, with an F- yield of 96% and a remaining TOC ratio of 2%.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Ventilation tubes (grommets) for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Nov 15;11(11):CD015215. doi: 10.1002/14651858.CD015215.pub2. Cochrane Database Syst Rev. 2023. PMID: 37965944 Free PMC article.
-
Adenoidectomy for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD015252. doi: 10.1002/14651858.CD015252.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870083 Free PMC article.
-
Antibiotics for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD015254. doi: 10.1002/14651858.CD015254.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870130 Free PMC article.
-
Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children.Cochrane Database Syst Rev. 2011 May 11;2011(5):CD001935. doi: 10.1002/14651858.CD001935.pub3. Cochrane Database Syst Rev. 2011. PMID: 21563132 Free PMC article.
-
Antibiotics for otitis media with effusion in children.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009163. doi: 10.1002/14651858.CD009163.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Jun 12;(6):CD009163. doi: 10.1002/14651858.CD009163.pub3. PMID: 22972136 Updated.
References
-
- Plueddemann, E. P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, 1991.
-
- Silanes and Other Coupling Agents, Mittal, K. L. , Ed.; VSP: Utrecht, 1992.
-
- Owen, M. J. ; Williams, D. E. . Surface modification by fluoroalkyl-functional silanes: a review. In Silanes and Other Coupling Agents, Mittal, K. L. , Ed.; VSP: Utrecht, 1992; pp 67–80.
-
- Organisation for Economic Co-operation and Development (OECD). Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs), Series on Risk Management No. 39, 2018.
LinkOut - more resources
Full Text Sources
