Evaluation of the analytical and clinical characteristics of the siemens immulite®2000 tsi method for determining thyrotropin receptor antibodies
- PMID: 40821630
- PMCID: PMC12357635
- DOI: 10.5937/jomb0-55873
Evaluation of the analytical and clinical characteristics of the siemens immulite®2000 tsi method for determining thyrotropin receptor antibodies
Abstract
Background: Despite commercially improved, standardised routine methods used in medical laboratories, precision laboratory medicine lacks harmonisation of results to make the laboratory result useful for its intended purpose. Furthermore, to obtain reliable laboratory results and precise diagnoses, it is important and recommended that each laboratory confirms the analytical and clinical characteristics of the method used. This study aimed to evaluate the analytical and clinical performance of the IMMULITE®2000 TSI bridge immunoassay to determine autoreactive thyroid stimulating hormone receptor antibodies (SH-R-Ab).
Methods: A total of 86 patients with clinically present Graves' orbitopathy and 23 healthy volunteers as a control group were included in the study. The total TSH-R-Ab concentration was determined using an ECLIA (Elecsys Anti-TSHR Immunoassay Roche Diagnostics, GmbH, Mannheim, Germany) on the Cobas e411 analyser (Roche, Diagnostics, GmbH). The TSH-R-Ab concentration was measured using a CLIA method (IMMULITE TSI 2000, Siemens Healthcare Diagnostics, UK). The inaccuracy of the method was investigated using two levels of commercial control samples (low and high analyte concentration).
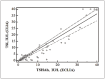
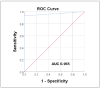
Results: The results obtained meet the general minimum requirements for the analytical performance of laboratory methods (CV<5%). The overall laboratory inaccuracy was acceptable according to FDA guidelines (CV<20%). The results showed a statistically significant correlation between the analysed methods (r=0.9041, p < 0.0001) but with a relative bias of 24.5%. The best ratio of sensitivity and specificity determined by the ROC analysis (93.3% and 100%, respectively) was obtained for a cut-off value of 0.1215 IU/L, which is significantly lower compared to the cut-off value specified by the manufacturer (0.55 IU/L).
Conclusions: The IMMULITE 2000 TSI bridge immunoassay for TSH-R-Ab quantification confirmed adequate precision, which is essential for routine use. However, further studies are required to evaluate its analytical specificity.
Uvod: Uprkos komercijalno poboljšanim, standardizovanim rutinskim metodama koje se koriste u medicinskim laboratorijama, preciznoj laboratorijskoj medicini nedostaje harmonizacija rezultata kako bi laboratorijski rezultat bio koristan za njegovu namenu. Dodatno, za dobijanje pouzdanih laboratorijskih rezultata i preciznijih dijagnoza važno je i preporučuje se da svaka laboratorija potvrdi analitičke i kliničke karakteristike primenjene metode. Cilj ove studije bio je da se procene analitičke i kliničke performanse IMMULITE®2000 TSI imunohemijskog testa za odre|ivanje autoreaktivnih antitela na receptore hormona koji stimulišu štitnu žlezdu (TSH-R-Ab).
Metode: U studiju je uključeno ukupno 86 bolesnika sa klinički prisutnom Gravesovom orbitopatijom i 23 zdrava dobro voljca kao kontrolna grupa. Ukupna koncentracija TSH-R-Ab određena je pomoću ECLIA (Elecsys Anti-TSHR Immunoassay Roche Diagnostics, GmbH, Mannheim, Germany) na analizatoru Cobas e411 (Roche, Diagnostics, GmbH). Koncentracija TSH-R-Ab merena je metodom CLIA (IMMULITE TSI 2000, Siemens Healthcare Diag - nostics, UK). Netačnost metode ispitana je pomoću dva nivoa komercijalnih kontrolnih uzoraka (niska i visoka koncentracija analita).
Rezultati: Dobijeni rezultati ispunjavaju opšte minimalne zahteve za analitičke performanse laboratorijskih metoda. (CV<5%). Ukupna laboratorijska netačnost bila je prihvatljiva prema smernicama FDA (CV<20%). Rezultati su pokazali statistički značajnu korelaciju izme|u analiziranih metoda (r=0,9041, p<0,0001), ali sa relativnim odstupanjem od 24,5%. Najbolji odnos osetljivosti i specifičnosti utvrđen ROC analizom (93,3% odnosno 100%) dobijen je za graničnu vrednost od 0,1215 IU/L, što je značajno niže u poređenju sa graničnom vrednošću koju je odredio proizvođač (0,55 IU/L).
Zaključak: IMMULITE 2000 TSI imunohemijski test za kvantifikaciju TSH-R-Ab potvrdio je adekvatan nivo preciznosti koji je neophodan za rutinsku upotrebu. Međutim, potrebna su dalja istraživanja kako bi se procenila njegova analitička specifičnost.
Keywords: analytical and clinical sensitivity and specificity; thyroid stimulating hormone receptor antibodies.
2025 Neda Milinković, Marija Sarić, Ana Ružanović, Miloš Žarković, Jasmina Ćirić, Blagojević Iva Perović, Ana-Marija Mastilović, Svetlana Ignjatović, Beleslin Biljana Nedeljković, published by CEON/CEES.
Conflict of interest statement
All the authors declare that they have no conflict of interest in this work.Conflict of Interest: The authors stated that they have no conflicts of interest regarding the publication of this article.
Figures
References
-
- Ehlers M, Schott M, Allelein S. Graves' disease in clinical perspective Front Biosci. 2019;24:35–47. - PubMed
LinkOut - more resources
Full Text Sources