Serum TNF-a and biomarkers levels after tenofovir therapy in patients with serum HBeAg-positive chronic hepatitis B
- PMID: 40821631
- PMCID: PMC12357611
- DOI: 10.5937/jomb0-56238
Serum TNF-a and biomarkers levels after tenofovir therapy in patients with serum HBeAg-positive chronic hepatitis B
Abstract
Background: Chronic hepatitis B (CHB) remains a wide-spread and serious infectious disease, with its pathogenesis still not fully understood. Tenofovir Amibufenamide, a pro-drug of tenofovir, belongs to the class of nucleoside reverse transcriptase inhibitors and is used in the treatment of CHB. This study aimed to evaluate the efficacy and safety of Tenofovir Amibufenamide in treating HBeAg-positive CHB.
Methods: A total of 60 patients diagnosed with HBeAg-positive CHB were randomly assigned to two groups: an entecavir (ETV) group (0.5 mg) and a Tenofovir Amibufenamide (TMF) group (0.25 mg), with 30 patients in each. After 24 months of treatment, renal function (serum creatinine, glomerular filtration rate), liver function (ALT , AST , total bilirubin), and inflammatory markers (TNF-a levels) were measured to assess the antiviral efficacy and safety profiles of the two treatments.
Results: Patients in the TMF group exhibited smaller changes in serum creatinine and glomerular filtration rate, indicating less renal impairment compared to the ETV group. Furthermore, the TMF group demonstrated greater reductions in alanine aminotransferase (ALT) and total bilirubin (TBIL) levels, indicating superior improvement in liver function (P<0.05). TNF-a levels were significantly elevated in the ETV group, reflecting greater inflammatory activity, while the TMF group showed more controlled inflammation. Additionally, the TMF group experienced significantly lower hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and HBV DNA levels, showing superior antiviral efficacy. The incidence of adverse reactions was lower in the TMF group (3.3%) compared to the ETV group (13.3%), although the difference was not statistically significant (P>0.05).
Conclusions: Tenofovir Amibufenamide demonstrated superior efficacy in improving liver function and reducing viral load in patients with HBeAg-positive CHB. Moreover, it had a minimal impact on renal function and presented a higher safety profile compared to entecavir. These findings suggest Tenofovir Amibufenamide as a promising alternative for the treatment of HBeAg-positive CHB.
Uvod: Hronični hepatitis B (CHB) i dalje predstavlja rasprostranjenu i ozbiljnu infektivnu bolest, čija patogeneza nije u potpunosti razjašnjena. Tenofovir amibufenamid, prekurzor tenofovira, pripada klasi inhibitora reverzne transkriptaze nukleozida i koristi se u lečenju CHB. Cilj ove studije bio je da se proceni efikasnost i bezbednost teno-fovir amibufenamida u lečenju HBeAg-pozitivnog CHB.
Metode: Ukupno 60 pacijenata sa dijagnozom HBeAg-pozitivnog CHB nasumično je podeljeno u dve grupe: grupa koja je primala entekavir (ETV) u dozi od 0,5 mg i grupa koja je primala tenofovir amibufenamid (TMF) u dozi od 0,25 mg, sa po 30 pacijenata u svakoj. Nakon 24 meseca terapije, procenjeni su parametri bubrežne funkcije (serum-ski kreatinin, glomerularna filtracija), funkcije jetre (ALT, AST, ukupni bilirubin) i inflamatorni markeri (nivoi TNF-a) kako bi se uporedila antivirusna efikasnost i bezbednosni profili dve terapije.
Rezultati: Pacijenti u TMF grupi imali su manje promene u nivoima serumskog kreatinina i glomerularnoj filtraciji, što ukazuje na manju bubrežnu disfunkciju u poređenju sa ETV grupom. Takođe, TMF grupa je pokazala veće smanjenje nivoa alanin aminotransferaze (ALT) i ukupnog biliru bina (TBIL), što ukazuje na superiorno poboljšanje funkcije jetre (P<0,05). Nivoi TNF-a bili su značajno povišeni u ETV grupi, što odražava veću inflamatornu aktivnost, dok je u TMF grupi upala bila bolje kontrolisana. Pored toga, u TMF grupi su zabeleženi značajno niži nivoi površinskog antigena hepatitisa B (HBsAg), antigena hepatitisa B e (HBeAg) i HBV DNK, što ukazuje na bolju antivirusnu efikasnost. Učestalost neželjenih reakcija bila je niža u TMF grupi (3,3%) u poređenju sa ETV grupom (13,3%), iako razlika nije bila statistički značajna (P>0,05).
Zaključak: Tenofovir amibufenamid je pokazao superiornu efikasnost u poboljšanju funkcije jetre i smanjenju virusnog opterećenja kod pacijenata sa HBeAg-pozitivnim CHB. Takođe, imao je minimalan uticaj na bubrežnu funkciju i povoljniji bezbednosni profil u poređenju sa entekavirom. Ovi nalazi ukazuju na to da tenofovir amibufenamid predstavlja obećavajuću alternativu za lečenje HBeAg-pozitivnog CHB.
Keywords: TNF-a; antiviral efficacy; chronic hepatitis B; entecavir; inflammatory markers; liver function; renal function; tenofovir amibufenamide.
2025 Xijie Lai, Guosheng Gao, Xiunong Jiang, published by CEON/CEES.
Conflict of interest statement
All the authors declare that they have no conflict of interest in this work.Conflict of Interest: The authors stated that they have no conflicts of interest regarding the publication of this article.
Figures

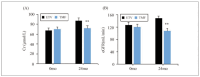

((A): AST; (B): ALT; and (C): TBIL).
Note: ** suggested a great difference with P<0.01 to the ETV group
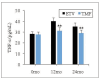
Note: * and ** suggested a great difference with P<0.05 and P<0.01 to the ETV group, respectively
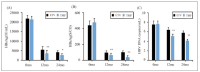
((A): HBsAg; (B): HBeAg; and (C): HBV DNA).
Note: * and ** suggested a great difference with P<0.05 and P<0.01 to ETV group, respectively
Similar articles
-
Tenofovir amibufenamide in chronic hepatitis B: Lipid changes and 144-week safety with tenofovir disoproxil fumarate-to-tenofovir amibufenamide switch.World J Gastroenterol. 2025 Jul 14;31(26):109285. doi: 10.3748/wjg.v31.i26.109285. World J Gastroenterol. 2025. PMID: 40678712 Free PMC article. Clinical Trial.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Safety and efficacy of GST-HG141, a novel HBV capsid assembly modulator, for the treatment of chronic hepatitis B patients with low-level viremia: a randomized, double-blind, placebo-controlled, multicenter phase II study.EClinicalMedicine. 2025 Aug 2;87:103400. doi: 10.1016/j.eclinm.2025.103400. eCollection 2025 Sep. EClinicalMedicine. 2025. PMID: 40808966 Free PMC article.
-
Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses.Gastroenterology. 2010 Oct;139(4):1218-29. doi: 10.1053/j.gastro.2010.06.042. Epub 2010 Jun 20. Gastroenterology. 2010. PMID: 20600036
-
Improvement in renal function after switching from entecavir to tenofovir alafenamide in chronic hepatitis B patients with low estimated glomerular filtration rates.Ann Hepatol. 2025 Jun 11;30(2):101925. doi: 10.1016/j.aohep.2025.101925. Online ahead of print. Ann Hepatol. 2025. PMID: 40513882
References
-
- Fatemipour M, Arabzadeh S A M, Molaei H, Geramizadeh B, Dabiri S, Fatemipour B, Vahedi S M, Malekpour Afshar R. Evaluation of STAT3 rs1053004 single nucleotide polymorphism in patients with chronic hepatitis B and hepatocellular carcinoma Cellular and Molecular Biology (Noisy-le-Grand, France) 2017;63(12):45. doi: 10.14715/10.14715/cmb/2017.63.12.11. - DOI - PubMed
-
- Wilkins T, Sams R, Carpenter M. Hepatitis B: screening, prevention, diagnosis, and treatment. American Family Physician. 2019;99(5):314. - PubMed
LinkOut - more resources
Full Text Sources
