Immunoglobulin A carries sulfated and O-acetylated N-glycans primarily at the tailpiece site - strategies for site-specific N-glycan identification
- PMID: 40821702
- PMCID: PMC12353717
- DOI: 10.3389/fmolb.2025.1595173
Immunoglobulin A carries sulfated and O-acetylated N-glycans primarily at the tailpiece site - strategies for site-specific N-glycan identification
Abstract
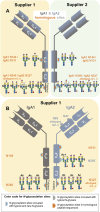
Sulfated N-glycans from human immunoglobulin A (IgA) were recently discovered via glycomic approaches. However, their site-specific description is still pending. Certain N-glycan structures at specific N-glycosylation sites in IgA are crucial for microbial neutralization and effector functions. For instance, sialylated N-glycans on the C-terminal tailpiece mediate anti-viral activity by interfering with sialic-acid-binding viruses. Sulfated N-glycan epitopes can be ligands for viral proteins and thus play a role in the immune response. In this study, we performed a site-specific screening for sulfated and other rare N-glycans in two commercially available human serum IgA samples employing an in-depth N-glycoproteomic approach, previously developed by us. We found evidence of complex-type and hybrid-type N-glycans containing sulfated N-acetylhexosamine (sulfated HexNAc) attached to the N-glycosylation sites in the tailpiece and the CH2 domain of both IgA subclasses. Also, complex-type N-glycan compositions bearing O-acetylated sialic acid were identified primarily at the tailpiece site. Surprisingly, N-glycans bearing glucuronic acid were identified in the commercial IgA samples, but from peptides of "contaminant" glycoproteins. A detailed comparison of the N-glycosylation profiles of human serum IgA samples from two suppliers showed such N-glycans with sulfated HexNAc consistently in higher abundance in the tailpiece region. These findings have not been described before for a site-specific glycopeptide analysis. Overall, our work provides strategies for performing a dedicated site-specific search for sulfated and O-acetylated N-glycans that can be easily transferred, e.g., to human IgA derived from mucosal tissues, milk, or saliva. We expect that a wider and deeper micro-heterogeneity description of clinically relevant glycoproteins, such as immunoglobulins, can expand the screening for biomarkers or treatment options.
Keywords: N-glycosylation; O-acetylated N-glycans; glycoproteomics; immunoglobulin A (IgA); mass spectrometry; oxonium marker ions; rare N-glycans; sulfated N-glycans.
Copyright © 2025 Zuniga-Banuelos, Lemke, Hoffmann, Reichl and Rapp.
Conflict of interest statement
ER is the founder and CEO of glyXera GmbH. FZ-B is an employee of glyXera GmbH and Max Planck Institute. glyXera provides high-performance glycoanalytical products and services, and holds several patents for xCGE-LIF-based glycoanalysis. UR is a shareholder of glyXera GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Alagesan K., Silva D. V., Seeberger P. H., Kolarich D. (2019). A novel, ultrasensitive approach for quantitative carbohydrate composition and linkage analysis using LC-ESI ion trap tandem mass spectrometry. bioRxiv. 10.1101/853036 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

