Dual targeting of BCMA and SLAMF7 with the CARtein system: chimeric antigen receptors with intein-mediated splicing elicit specific T cell activation against multiple myeloma
- PMID: 40821776
- PMCID: PMC12350263
- DOI: 10.3389/fimmu.2025.1613222
Dual targeting of BCMA and SLAMF7 with the CARtein system: chimeric antigen receptors with intein-mediated splicing elicit specific T cell activation against multiple myeloma
Abstract
Introduction: Chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy against multiple myeloma (MM). However, several barriers continue to limit the overall effectiveness of this approach, such as high production costs, prolonged manufacturing timelines, safety issues, and the potential for tumor antigen escape due to selective therapeutic pressure. To overcome these challenges, innovative CAR T strategies, such as engineering modular CAR systems, are being explored. These systems utilize adaptor molecules to enable multi-antigen targeting, thereby enhancing specificity, safety, and overall efficiency of CAR T-cell therapy. Notably, CAR T-cells directed against BCMA and SLAMF7 antigens have generated strong and robust antitumor responses in MM therapy.
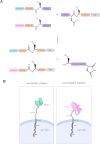
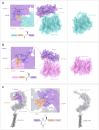
Methods: To address the limitations of conventional CAR T therapy, we developed a novel modular CAR platform targeted against BCMA and SLAMF7. This was achieved using a split intein-mediated protein splicing mechanism, which allows specific covalent peptide bonds to form between CAR modules. This strategy maintains an almost seamless CAR structure, preserving its overall integrity and functionality. The design of the intein-spliced CAR system (termed "CARtein") was further optimized through advanced protein structure prediction software.
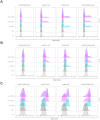
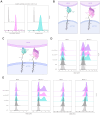
Results: Cells expressing the spliced CARtein constructs, engineered to target BCMA, SLAMF7, or both antigens simultaneously, demonstrated robust and highly specific activation in response to their respective antigens.
Discussion: These results suggest that the CARtein platform is a promising, versatile, and highly specific approach for the modular design and engineering of CARs, enabling multi-antigen targeting while maintaining structural and functional integrity. This modular strategy addresses key limitations of conventional CAR T-cell therapy and may improve both the safety and effectiveness of future MM treatments.
Keywords: B-cell maturation antigen; SLAMF7; chimeric antigen receptor; immunotherapy; inteins; modular CAR; multiple myeloma; protein splicing.
Copyright © 2025 Moares, Gonzalez-Garcia, Yi-He, Muñoz-Miranda, Gabucio, Luna-Espejo, Ocaña-Cuesta, Fernandez-Cisnal, Fernandez-Ponce and Garcia-Cozar.
Conflict of interest statement
All authors are inventors of the patent application EP25382080.7, filed by the University of Cádiz and INIBICA. The patent pertains to modular CARs, and uses thereof, which is directly related to the findings reported in this manuscript. No financial or non-financial benefits have been realized from this patent at the time of publication. The authors confirm that no other competing interests exist.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

